Media Release
March 2021
Chemistry in Australia
 Charge up your photoinitiators
Oriented electric fields can influence chemical reactivity and recent research is applying this idea to enhance the performance of photoinitiators. Importantly for photoinitiators, shifts in the absorption transition must be considered along with shifts of other excited states that mediate activation (e.g. α-cleavage bond homolysis). Researchers at the University of Wollongong and Australian National University have shown how oriented electric fields, arising from single monatomic cations, can be used to tune the photodissociation of a common photoinitiator, Irgacure 2959.
Charge up your photoinitiators
Oriented electric fields can influence chemical reactivity and recent research is applying this idea to enhance the performance of photoinitiators. Importantly for photoinitiators, shifts in the absorption transition must be considered along with shifts of other excited states that mediate activation (e.g. α-cleavage bond homolysis). Researchers at the University of Wollongong and Australian National University have shown how oriented electric fields, arising from single monatomic cations, can be used to tune the photodissociation of a common photoinitiator, Irgacure 2959.
Electrostatically Tuning the Photodissociation of the Irgacure 2959 Photoinitiator in the Gas Phase by Cation Binding J. Am. Chem. Soc., (2021), 143, 2331-2339. https://dx.doi.org/10.1021/jacs.0c11978
Chemistry in Australia
 Electrified bubbles
The formation of bubbles at electrodes is a ubiquitous problem in technologies from batteries to industrial smelting. An adhering gas bubble will mask a portion of the electrode, preventing fresh solution from reaching it. Consequently, electrochemists and engineers unanimously regard surface bubbles as redox-inactive passivating entities. But now a team of researchers from Curtin University, the Australian National University, the University of New South Wales and the University of Western Australia has demonstrated that this is not case: bubbles adhering to an electrode surface initiate the oxidation of water-soluble species under conditions for which such reactions would normally be considered impossible.
Electrified bubbles
The formation of bubbles at electrodes is a ubiquitous problem in technologies from batteries to industrial smelting. An adhering gas bubble will mask a portion of the electrode, preventing fresh solution from reaching it. Consequently, electrochemists and engineers unanimously regard surface bubbles as redox-inactive passivating entities. But now a team of researchers from Curtin University, the Australian National University, the University of New South Wales and the University of Western Australia has demonstrated that this is not case: bubbles adhering to an electrode surface initiate the oxidation of water-soluble species under conditions for which such reactions would normally be considered impossible.
The Corona of a Surface Bubble Promotes Electrochemical Reactions Nat. Comm., (2020), 11, 6323. https://doi.org/10.1038/s41467-020-20186-0
September 2020
Chemistry in Australia
 Electrostatic Catalysis by Solvent Ordering
External electric fields have recently been shown to catalyse chemical reactions, but scaling these effects in practical experimental systems has been elusive. Now researchers from the ANU and Monash University have shown that ordered solvent environments can provide a way forward. This work takes advantage of the ability of solvents and ionic liquids to become ordered under external electric fields and, in the case of ionic liquids, to maintain that order for some time after the field is removed. This ordered solvent environment generates its own internal electric field that can be exploited for catalysis.
Electrostatic Catalysis by Solvent Ordering
External electric fields have recently been shown to catalyse chemical reactions, but scaling these effects in practical experimental systems has been elusive. Now researchers from the ANU and Monash University have shown that ordered solvent environments can provide a way forward. This work takes advantage of the ability of solvents and ionic liquids to become ordered under external electric fields and, in the case of ionic liquids, to maintain that order for some time after the field is removed. This ordered solvent environment generates its own internal electric field that can be exploited for catalysis.
Ordered Solvents and Ionic Liquids Can be Harnessed for Electrostatic Catalysis J. Am. Chem. Soc., (2020), 142, 29, 12826-12833. https://dx.doi.org/10.1021/jacs.0c05643
Chemistry World
 The quest to control chemical reactions using interfacial electric fields
Electrostatic interactions underlie all of chemistry. Beyond the complicated chemical equations and reaction schemes that are used to describe chemical reactions, it is the rearrangement of charges and their associated electric fields that govern chemical reactivity. This is the conceptual understanding from which we derive our electron-pushing mechanisms and rationalise how we go from starting materials to products. Given that the probability and mechanism by which a reactant species is transformed into product is so dependent on the nature of the associated electric fields, it is not surprising that modulating the electric field environment surrounding a reacting system can influence its outcome.
The quest to control chemical reactions using interfacial electric fields
Electrostatic interactions underlie all of chemistry. Beyond the complicated chemical equations and reaction schemes that are used to describe chemical reactions, it is the rearrangement of charges and their associated electric fields that govern chemical reactivity. This is the conceptual understanding from which we derive our electron-pushing mechanisms and rationalise how we go from starting materials to products. Given that the probability and mechanism by which a reactant species is transformed into product is so dependent on the nature of the associated electric fields, it is not surprising that modulating the electric field environment surrounding a reacting system can influence its outcome.
August 2020
New Scientist
 Can static electricity make chemistry more efficient - and greener?
IT ISN’T long after waking each day that we meet the handiwork of chemists. The flavourings in toothpaste, scents in shower gel, polyester in clothes – all have been created through the breaking and making of chemical bonds. The same goes for nearly all the materials on which the modern world relies. It isn’t easy work. Take remdesivir, the antiviral drug that could help us treat Covid-19. To make it, chemists begin with a small molecule called alanine and add a further 64 atoms to it over the course of 25 separate chemical reactions. Whew. Making such molecular marvels isn’t just taxing, it can also be a grubby affair. Synthetic chemists spend most of their time amid pastes, powders and bubbling solutions: it is a messy and often smelly craft. But perhaps there is a way to make it simpler and cleaner. More and more chemists are experimenting with a new tool of subtle power: the electric field. Not only does it promise to help us less damaging to the environment. If this works, chemistry will be transformed.
Can static electricity make chemistry more efficient - and greener?
IT ISN’T long after waking each day that we meet the handiwork of chemists. The flavourings in toothpaste, scents in shower gel, polyester in clothes – all have been created through the breaking and making of chemical bonds. The same goes for nearly all the materials on which the modern world relies. It isn’t easy work. Take remdesivir, the antiviral drug that could help us treat Covid-19. To make it, chemists begin with a small molecule called alanine and add a further 64 atoms to it over the course of 25 separate chemical reactions. Whew. Making such molecular marvels isn’t just taxing, it can also be a grubby affair. Synthetic chemists spend most of their time amid pastes, powders and bubbling solutions: it is a messy and often smelly craft. But perhaps there is a way to make it simpler and cleaner. More and more chemists are experimenting with a new tool of subtle power: the electric field. Not only does it promise to help us less damaging to the environment. If this works, chemistry will be transformed.
July 2020
Chemistry in Australia
 Enzyme-inspired self-assembling surfactant catalysts
Enzymes carry out their life-giving reactions by gathering together a remarkably intricate arrangement of functional groups. It is the precisely defined protein structure of enzymes that establishes these interactions. However, it is also this protein structure that makes enzymes susceptible to deactivation by heat, salts, and organic solvents. This problem has sparked interest in the design of enzyme-inspired catalysts that collect complimentary functionalities without relying on delicate protein asssemblies. A diverse team of researchers from the Australian National University and the University of Melbourne has recently developed a new, enzyme-inspired catalyst system that employs multi-functional surfactants to carry out catalysis.
Enzyme-inspired self-assembling surfactant catalysts
Enzymes carry out their life-giving reactions by gathering together a remarkably intricate arrangement of functional groups. It is the precisely defined protein structure of enzymes that establishes these interactions. However, it is also this protein structure that makes enzymes susceptible to deactivation by heat, salts, and organic solvents. This problem has sparked interest in the design of enzyme-inspired catalysts that collect complimentary functionalities without relying on delicate protein asssemblies. A diverse team of researchers from the Australian National University and the University of Melbourne has recently developed a new, enzyme-inspired catalyst system that employs multi-functional surfactants to carry out catalysis.
A Multi-Functional Surfactant Catalyst Inspired by Hydrolases Science Advances (2020), 6, 14, eaaz0404. https://dx.doi.org/10.1126/sciadv.aaz0404
March 2020
Chemistry in Australia
 Electric Fields Cooperatively Promote Ground and Excited State Reactivity
The demonstration that electric fields can be used to catalyse and control the reactivity of chemical reactions has recently sparked immense interest, with both theoreticians and experimentlaists exploring a range or avenues for harnessing these effects. Now, researchers from the Australian National University have shown that electric fields from remote charged functional groups can selectively and cooperatively promote both ground- and excited-state reactivity in the same system.
Electric Fields Cooperatively Promote Ground and Excited State Reactivity
The demonstration that electric fields can be used to catalyse and control the reactivity of chemical reactions has recently sparked immense interest, with both theoreticians and experimentlaists exploring a range or avenues for harnessing these effects. Now, researchers from the Australian National University have shown that electric fields from remote charged functional groups can selectively and cooperatively promote both ground- and excited-state reactivity in the same system.
Oriented Internal Electrostatic Fields Cooperatively Promote Ground & Excited State Reactivity: A Case Study in Photochemical CO2 Capture J. Am. Chem. Soc. (2020), 142, 606-613. https://dx.doi.org/10.1021/jacs.9b12186
November 2019
Chemistry in Australia
 Electrophilic methylating agent activated by electrochemistry
Alkoxyamines are important compounds in polymer synthesis due to their ability to form a persistent nitroxide and a carboncentred radical at elevated temperatures, thus facilitating controlled radical polymerisation. However, using a combination of experimental and computational chemistry, researchers at the Australian National University recently showed that, by electrochemically oxidising these molecules, they can instead generate carbocations.
Electrophilic methylating agent activated by electrochemistry
Alkoxyamines are important compounds in polymer synthesis due to their ability to form a persistent nitroxide and a carboncentred radical at elevated temperatures, thus facilitating controlled radical polymerisation. However, using a combination of experimental and computational chemistry, researchers at the Australian National University recently showed that, by electrochemically oxidising these molecules, they can instead generate carbocations.
TEMPO–Me: An Electrochemically Activated Methylating Agent J. Am. Chem. Soc., (2019) 141, 38, 15450-15455. http://dx.doi.org/10.1021/jacs.9b08634
Chemistry in Australia
 Stable contacts for molecular electronics
Single molecules are predicted to play a key role in the future of miniaturised electronics. One of the biggest challenges facing molecular electronics today is the lack of mechanically stable single molecule contacts to metal or semiconducting electrodes. In a study led by Nadim Darwish at Curtin University, single molecules, terminated by diazonium salts at both ends, were used to form covalent bonds to both gold and silicon electrodes, mimicking standard metal–insulator–semiconductor diodes
Metal‒single-molecule‒semiconductor junctions formed by a radical reaction bridging gold and silicon electrodes
J. Am. Chem. Soc., (2019) 141, 38, 14788−14797.
http://dx.doi.org/10.1021/jacs.9b07125
Stable contacts for molecular electronics
Single molecules are predicted to play a key role in the future of miniaturised electronics. One of the biggest challenges facing molecular electronics today is the lack of mechanically stable single molecule contacts to metal or semiconducting electrodes. In a study led by Nadim Darwish at Curtin University, single molecules, terminated by diazonium salts at both ends, were used to form covalent bonds to both gold and silicon electrodes, mimicking standard metal–insulator–semiconductor diodes
Metal‒single-molecule‒semiconductor junctions formed by a radical reaction bridging gold and silicon electrodes
J. Am. Chem. Soc., (2019) 141, 38, 14788−14797.
http://dx.doi.org/10.1021/jacs.9b07125
October 2019
ABC Science
 Is a substitute for plastic coming anytime soon?
For most of us, hearing the world plastic makes us think of single-use plastics like plastic bags or disposable plastic water bottles, but plastic covers a much wider variety of materials than that.
Is a substitute for plastic coming anytime soon?
For most of us, hearing the world plastic makes us think of single-use plastics like plastic bags or disposable plastic water bottles, but plastic covers a much wider variety of materials than that.
July 2019
Chemistry in Australia
 Getting a Grip on Static Electricity
Electrically insulating objects gain a net electrical charge when brought into and out of contact. This phenomenon – triboelectricity – involves the flow of charged species, but to conclusively establish their nature has proven extremely difficult. A team of researchers from Curtin University, the Australian National University and the University of New South Wales has studied the redox growth of metal nanoparticles on electrostatically charged polymers... they have described for the first time, a material-specific relationship between the tribocharging magnitude and the extent of redox word that can be harvested from tribocharged polymers.
Getting a Grip on Static Electricity
Electrically insulating objects gain a net electrical charge when brought into and out of contact. This phenomenon – triboelectricity – involves the flow of charged species, but to conclusively establish their nature has proven extremely difficult. A team of researchers from Curtin University, the Australian National University and the University of New South Wales has studied the redox growth of metal nanoparticles on electrostatically charged polymers... they have described for the first time, a material-specific relationship between the tribocharging magnitude and the extent of redox word that can be harvested from tribocharged polymers.
Electrochemistry on Tribocharged Polymers Is Governed by the Stability of Surface Charges Rather than Charging Magnitude J. Am. Chem. Soc., (2019) 141, 5863-5870. http://dx.doi.org/10.1021/jacs.9b00297
May 2019
Chemistry in Australia
 Australian Journal of Chemistry's 70th Birthday Edition
To celebrate its 70th birthday, the Australian Journal of Chemistry has released a special online collection of published papers. CSIRO Publishing’s Jennifer Foster highlights some of the selections, including a "landmark paper by Michelle Coote and co-workers, (Aust. J. Chem. 2005, 58, 437-441) describing a viable synthetic route to a novel class of RAFT agents bearing a fluorine Z-group."
Australian Journal of Chemistry's 70th Birthday Edition
To celebrate its 70th birthday, the Australian Journal of Chemistry has released a special online collection of published papers. CSIRO Publishing’s Jennifer Foster highlights some of the selections, including a "landmark paper by Michelle Coote and co-workers, (Aust. J. Chem. 2005, 58, 437-441) describing a viable synthetic route to a novel class of RAFT agents bearing a fluorine Z-group."
March 2019
Chemistry in Australia
 Altering photochemistry with static electric fields
It has recently been shown that static electric fields can be used to catalyse non-electrochemical reactions, opening up a new approach to chemical catalysis. Now, researchers at the Australian National University have expanded electrostatic catalysis into the realm of molecular excited states. Using state-of- the-art computational methods, they have shown that static electric fields can be used as an approach to modifying the relative energies of different types of excited state in a predictable and significant manner.
Altering photochemistry with static electric fields
It has recently been shown that static electric fields can be used to catalyse non-electrochemical reactions, opening up a new approach to chemical catalysis. Now, researchers at the Australian National University have expanded electrostatic catalysis into the realm of molecular excited states. Using state-of- the-art computational methods, they have shown that static electric fields can be used as an approach to modifying the relative energies of different types of excited state in a predictable and significant manner.
Hill, N.S.; Coote, M.L., Internal Oriented Electric Fields as a Strategy for Selectively Modifying Photochemical Reactivity J. Am. Chem. Soc., (2018) 140, 17800−17804. http://dx.doi.org/10.1021/jacs.8b12009
January 2019
Chemistry in Australia
 Discrete and stereospecific oligomers as natural biopolymer mimics
Natural biopolymers such as DNA and proteins have uniform microstructures with defined molecular weight, precise monomer sequence, and stereoregularity along the polymer main chain, which endows them with unique biological functions. Mimicking natural biopolymers, Jiangtao (Jason) Xu and co-workers at the University of New South Wales and their collaborators from the Australian National University, CSIRO, University of California, Santa Barbara, USA, and Nagoya University, Japan, recently established a new method to prepare discrete and stereospecific oligomers using sequential and alternating photoinduced RAFT single-unit monomer insertion (Photo-RAFT SUMI).
Discrete and stereospecific oligomers as natural biopolymer mimics
Natural biopolymers such as DNA and proteins have uniform microstructures with defined molecular weight, precise monomer sequence, and stereoregularity along the polymer main chain, which endows them with unique biological functions. Mimicking natural biopolymers, Jiangtao (Jason) Xu and co-workers at the University of New South Wales and their collaborators from the Australian National University, CSIRO, University of California, Santa Barbara, USA, and Nagoya University, Japan, recently established a new method to prepare discrete and stereospecific oligomers using sequential and alternating photoinduced RAFT single-unit monomer insertion (Photo-RAFT SUMI).
Huang, Z.; Noble, B.B.; Corrigan, N.; Chu, Y.; Satoh, K.; Thomas, D.; Hawker, C.J.; Moad, G.; Kamigaito, M.; Coote, M.L.; Boyer, C.; Xu, J., Discrete and Stereospecific Oligomers Prepared by Sequential and Alternating Single Unit Monomer Insertion J. Am. Chem. Soc., (2018) 140, 13392-13406. http://dx.doi.org/10.1021/jacs.8b08386
July 2018
Eureka Prize for Scientific Research Finalists Announced
 The Invisible Catalyst Team
Developing efficient ways to catalyse reactions has been an important quest for scientific research. The Invisible Catalyst Team, Professor Michelle Coote, Dr Simone Ciampi and Dr Nadim Darwish, has shown that electric fields can be used to manipulate chemical reactions. This breakthrough may enable greener and safer methods for fabricating materials, from drugs to plastics.
The Invisible Catalyst Team
Developing efficient ways to catalyse reactions has been an important quest for scientific research. The Invisible Catalyst Team, Professor Michelle Coote, Dr Simone Ciampi and Dr Nadim Darwish, has shown that electric fields can be used to manipulate chemical reactions. This breakthrough may enable greener and safer methods for fabricating materials, from drugs to plastics.
April 2018
Chemistry in Australia
 Triggering bond cleavage with electric fields
Electricity has long been used in chemistry to trigger electrochemical reactions, but only recently have static electric fields been shown to catalyse non-electrochemical reactions. But implementation has to date required scanning tunnelling microscopy (STM) to orient the reagents appropriately in the electric field. Now, a team of researchers from Curtin University, the Australian National University, the University of Wollongong, ANSTO, the Silesian University of Technology, Poland, and the University of Murcia, Spain, has shown that electrostatic factors contribute to the catalysis of a chemical process that follows an anodic reaction in an electrochemical cell.
Triggering bond cleavage with electric fields
Electricity has long been used in chemistry to trigger electrochemical reactions, but only recently have static electric fields been shown to catalyse non-electrochemical reactions. But implementation has to date required scanning tunnelling microscopy (STM) to orient the reagents appropriately in the electric field. Now, a team of researchers from Curtin University, the Australian National University, the University of Wollongong, ANSTO, the Silesian University of Technology, Poland, and the University of Murcia, Spain, has shown that electrostatic factors contribute to the catalysis of a chemical process that follows an anodic reaction in an electrochemical cell.
Zhang, L.; Laborda, E.; Darwish, N.; Noble, B.B.; Tyrell, J.H.; Pluczyk, S.; Le Brun, A.P.; Wallace, G.G.; Gonzalez, J.; Coote, M.L.; Ciampi, S., Electrochemical and Electrostatic Cleavage of Alkoxyamines J. Am. Chem. Soc., (2018) 140, 766−774. http://dx.doi.org/10.1021/jacs.7b11628
 When electrochemical measurement artefacts are real
Researchers from Curtin University, the University of Murcia, Spain, the University of Wollongong, the Australian National University and the University of New South Wales have been able to reproduce and explain the often puzzling behaviour of electrons that enter or leave semiconductor materials.
When electrochemical measurement artefacts are real
Researchers from Curtin University, the University of Murcia, Spain, the University of Wollongong, the Australian National University and the University of New South Wales have been able to reproduce and explain the often puzzling behaviour of electrons that enter or leave semiconductor materials.
Vogel, Y.B.; Zhang, L.; Darwish, N.; Gonçales, V.R.; Le Brun, A.; Gooding, J.J.; Molina, A.; Wallace, G.G.; Coote, M.L.; Gonzalez, J.; Ciampi, S., Reproducible flaws unveil electrostatic aspects of semiconductor electrochemistry Nature Comm., (2017) 8, 2066. http://dx.doi.org/10.1038/s41467-017-02091-1
February 2018
Chemistry in Australia
Light, reactivity, action!
Efficient light-induced ligation protocols are valuable tools for functional materials design. The teams of Christopher Barner-Kowollik and James Blinco, at the Queensland University of Technology and the Karlsruhe Institute of Technology, Germany, and Michelle Coote at the Australian National University have investigated two highly efficient photoligation reactions involving photoenols and nitrile imines in a combined experimental and theoretical study
Menzel, J.P.; Noble, B.B.; Lauer, A.; Coote, M.L.; Blinco, J.P.; Barner-Kowollik, C. Wavelength Dependence of Light-Induced Cycloadditions J. Am. Chem. Soc., (2017) 139, 15812-15820. http://dx.doi.org/10.1021/jacs.7b08047
January 2018
Chemistry World
 Field of Influence
Chemists are used to harnessing all sorts of subtle and not-so-subtle tools to choreograph the dance of molecules, from lasers to microwaves to plain old heating and stirring. But now, in a few labs around the world, an unusual new idea is crackling and sparking into life: chemists are starting to explore whether electric fields can be used to control reactions too.
Field of Influence
Chemists are used to harnessing all sorts of subtle and not-so-subtle tools to choreograph the dance of molecules, from lasers to microwaves to plain old heating and stirring. But now, in a few labs around the world, an unusual new idea is crackling and sparking into life: chemists are starting to explore whether electric fields can be used to control reactions too.
Chemical and Engineering News
 What will be chemistry's next big thing?
What will be chemistry's next big thing?
Michelle Coote, professor, Research School of Chemistry, Australian National University; associate editor, Journal of the American Chemical Society
What? Electric fields as catalysts
Why? The use of electric fields as catalysts is not yet commonplace. The first paper demonstrating the process in a nonredox reaction was published in 2016 in Nature (DOI: 10.1038/nature16989). “But I think the potential is enormous and we are just starting to explore it,” Coote says. “The timing is also fantastic, as the use of electrochemistry to trigger chemical reactions is also starting to gain traction as a routine tool for organic synthesis.”
December 2017
Chemistry in Australia
 Electrical Mechanochemistry
Normally one assumes that the redox potential of a molecule is a unique property of the molecule, its solvent environment and the temperature. However, researchers from the Arizona State University, The Australian National University and Curtin University have now demonstrated that mechanical force can also be used to manipulate electron transfer reactions.
Electrical Mechanochemistry
Normally one assumes that the redox potential of a molecule is a unique property of the molecule, its solvent environment and the temperature. However, researchers from the Arizona State University, The Australian National University and Curtin University have now demonstrated that mechanical force can also be used to manipulate electron transfer reactions.
Li, Y.; Haworth, N.L.; Xiang, L.; Ciampi, S.; Coote, M.L.; Tao, N.J. Mechanical stretching-induced electron transfer reactions and conductance switching in single molecules J. Am. Chem. Soc., (2017) 139, 14699-14706. http://dx.doi.org/10.1021/jacs.7b08239
September 2017
Chemistry in Australia
 2017 ARC Laureate Fellowships announced
Professor Michelle Coote FRACI CChem (pictured) from the ANU Research School of Chemistry has received the Georgina Sweet Australian Laureate Fellowship for science and technology for a project to establish a new approach to chemical catalysis.
2017 ARC Laureate Fellowships announced
Professor Michelle Coote FRACI CChem (pictured) from the ANU Research School of Chemistry has received the Georgina Sweet Australian Laureate Fellowship for science and technology for a project to establish a new approach to chemical catalysis.
October 2016
Chemistry in Australia
 Controlling electroactivity of surface-tethered radicals
Cyclic voltammetry is a well-established form of electrochemical ‘spectroscopy’ that yields a great wealth of mechanistic and thermodynamic information from the analysis of a current flowing across an electrified interface. A team of researchers from the University of Wollongong, the Australian National University, the University of New South Wales, Institut de Bioenginyeria de Catalunya and ANSTO has used this technique to show how a seemingly simple ‘dynamic’ current−potential trace can yield quantitative insights into the ‘electrostatic’ environment around a surface- tethered nitroxide radical (Zhang L., Vogel Y.B., Noble B.B., Gonçales V.R., Darwish N., Le Brun A., Gooding J.J., Wallace G.G., Coote M.L., Ciampi S. J. Am. Chem. Soc. 2016, 138, 9611−9). The level of doping and nature of the electrolyte were found to cause drastic kinetic changes to the electroactivity of the radical monolayer as well as electrochemical non-idealities. Calculations indicate that these unusual effects are electrostatic in origin and arise from interactions between the nitroxide radical (2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO)) and either electrolyte species or ionised dopants in the semiconducting electrode. This work has important implications for how charged groups or externally applied electric fields can influence chemical bonding and reactivity, an area that is beginning to attract enormous interest (see, for example, Aragonès A.C. et al., Nature 2016, 531, 88−91).
Controlling electroactivity of surface-tethered radicals
Cyclic voltammetry is a well-established form of electrochemical ‘spectroscopy’ that yields a great wealth of mechanistic and thermodynamic information from the analysis of a current flowing across an electrified interface. A team of researchers from the University of Wollongong, the Australian National University, the University of New South Wales, Institut de Bioenginyeria de Catalunya and ANSTO has used this technique to show how a seemingly simple ‘dynamic’ current−potential trace can yield quantitative insights into the ‘electrostatic’ environment around a surface- tethered nitroxide radical (Zhang L., Vogel Y.B., Noble B.B., Gonçales V.R., Darwish N., Le Brun A., Gooding J.J., Wallace G.G., Coote M.L., Ciampi S. J. Am. Chem. Soc. 2016, 138, 9611−9). The level of doping and nature of the electrolyte were found to cause drastic kinetic changes to the electroactivity of the radical monolayer as well as electrochemical non-idealities. Calculations indicate that these unusual effects are electrostatic in origin and arise from interactions between the nitroxide radical (2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO)) and either electrolyte species or ionised dopants in the semiconducting electrode. This work has important implications for how charged groups or externally applied electric fields can influence chemical bonding and reactivity, an area that is beginning to attract enormous interest (see, for example, Aragonès A.C. et al., Nature 2016, 531, 88−91).
Zhang, L.; Vogel, Y.B.; Noble, B.B.; Gonçales, V.R.; Darwish, N.; Le Brun, A.; Gooding, J.J.; Wallace, G.G.; Coote, M.L; Ciampi, S. TEMPO monolayers on Si(100) electrodes; electrostatic effects by the electrolyte and semiconductor space-charge on the electroactivity of a persistent radical J. Am. Chem. Soc., (2016) 38, 9611–9619.http://dx.doi.org/10.1021/jacs.6b04788
April 2016
Chemistry in Australia
 Where macromolecules cleave: entropic selectivity for chain scission
Reversible covalent and supramolecular bonding is increasingly being employed in applications such as self-healing and stimuli-responsive materials, complex macromolecular architectures and protein mimics. Normally, dynamic ligation equilibria are tuned by modifying the ligating functional groups to alter their electronic properties and thus reaction enthalpy. However, researchers from the Karlsruhe Institute of Technology, the Australian National University and the Leibniz Institute for Polymer Research have demonstrated that the equilibria can be tuned by altering the molecular weight and chain stiffness of the linking groups without changing the actual bonding motifs, thus taking advantage of their effect on entropy instead of enthalpy. They showed that an important consequence of these entropic effects is a significant preference for cleavage of macromolecules in the middle of long chains, rather than at the ends (Pahnke K., Brandt J., Gryn’ova G., Lin C.Y., Altintas O., Schmidt F.G., Lederer A., Coote M.L., Barner-Kowollik C., Angew. Chem. Int. Ed. 2016, 55, 1514-18). The results were predicted via model calculations and confirmed via experimental analysis of reversible covalent and supramolecular adducts. An important consequence of the work is the prediction of long-range chain length effects on chemically controlled polymer-polymer reactions such as chain transfer, polymer degradation, and control agent addition.
Where macromolecules cleave: entropic selectivity for chain scission
Reversible covalent and supramolecular bonding is increasingly being employed in applications such as self-healing and stimuli-responsive materials, complex macromolecular architectures and protein mimics. Normally, dynamic ligation equilibria are tuned by modifying the ligating functional groups to alter their electronic properties and thus reaction enthalpy. However, researchers from the Karlsruhe Institute of Technology, the Australian National University and the Leibniz Institute for Polymer Research have demonstrated that the equilibria can be tuned by altering the molecular weight and chain stiffness of the linking groups without changing the actual bonding motifs, thus taking advantage of their effect on entropy instead of enthalpy. They showed that an important consequence of these entropic effects is a significant preference for cleavage of macromolecules in the middle of long chains, rather than at the ends (Pahnke K., Brandt J., Gryn’ova G., Lin C.Y., Altintas O., Schmidt F.G., Lederer A., Coote M.L., Barner-Kowollik C., Angew. Chem. Int. Ed. 2016, 55, 1514-18). The results were predicted via model calculations and confirmed via experimental analysis of reversible covalent and supramolecular adducts. An important consequence of the work is the prediction of long-range chain length effects on chemically controlled polymer-polymer reactions such as chain transfer, polymer degradation, and control agent addition.
Pahnke, K.; Brandt, J.; Gryn’ova, G.; Lin, C.Y.; Altintas, O.; Schmidt, F.G.; Lederer, A.; Coote, M.L.; Barner-Kowollik, C., Entropy-Driven Selectivity for Chain Scission: Where Macromolecules Cleave Angew. Chem., (2016) 55, 1514-1518.http://dx.doi.org/10.1002/anie.201508531
March 2016
Chemical and Engineering News
 In a discovery that might come as a shock—or, at the very least, an electric shock—chemists have found that a properly oriented external electric field can nudge two reagents to hook up with one another in a Diels-Alder reaction. The fundamental discovery expands chemists’ knowledge of how electricity can drive synthesis and catalysis.
In a discovery that might come as a shock—or, at the very least, an electric shock—chemists have found that a properly oriented external electric field can nudge two reagents to hook up with one another in a Diels-Alder reaction. The fundamental discovery expands chemists’ knowledge of how electricity can drive synthesis and catalysis.
Chemists have long used electricity to trigger redox reactions. And theorists have suggested that electric fields could spur on non-redox transformations, but until now, no one had shown this was possible with a bimolecular system. “What is particularly striking is that we chose a really simple nonpolar carbon-carbon-bond-forming reaction—a Diels-Alder reaction—for which there are no formal zwitterionic intermediates involved,” says Michelle L. Coote, a professor at Australian National University who coauthored the study. “So we think these electric field effects could be very general.”
Chemistry World
 Scientists in Australia and Spain have shown electrostatic fields can speed up simple carbon—carbon bond-forming reactions,1 overturning a long-held chemical assumption. Michelle Coote from the Australian National University in Canberra and her coworkers changed reaction rate with the flick of a switch, giving organic chemists a potentially powerful new tool.
Scientists in Australia and Spain have shown electrostatic fields can speed up simple carbon—carbon bond-forming reactions,1 overturning a long-held chemical assumption. Michelle Coote from the Australian National University in Canberra and her coworkers changed reaction rate with the flick of a switch, giving organic chemists a potentially powerful new tool.
Electric fields are known to influence redox reactions that involve changes in an atom’s oxidation state, but hadn’t been thought to have a role beyond that, Coote says. ‘A key empirical rule in chemistry is that only temperature and concentration affect the rate of non-redox reactions,’ she adds. ‘We now believe these field effects would be almost universal, though with differing sensitivities. We deliberately chose a relatively non-sensitive reaction to demonstrate this.’
See also
Nature
Aragonès, A.C.; Haworth, N.L.; Darwish, N.; Ciampi, S.; Bloomfield, N.J.; Wallace, G.G.; Diez-Perez, I.; Coote, M.L., Electrostatic catalysis of a Diels-Alder reaction, Nature (2016) 531, 88-91. http://dx.doi.org/10.1038/nature16989
Nature News and Views
Xiang, L.; Tao, N.J., Reactions triggered by electricity, Nature (2016) 531, 38-39. http://dx.doi.org/10.1038/531038a
March 2016
Chemistry in Australia
 Chemoselective switch in asymmetric organocatalysis
Most chemical reactions are straightforward and furnish only one set of products. However, a holy grail in synthesis is to design reactions that can be readily ‘tuned’ to deliver different product sets that can be selected according to the reaction conditions, reagents, catalysts and even post purification strategies. This task is even more challenging when enantio- and diastereo- discrimination is required. Collaborative work by researchers from Henan University and Australian National University (Zhu, B.; Lee, R.; Li, J.; Ye, X.; Hong, S.-N.; Qiu, S.; Coote, M.L.; Jiang, Z., Angew. Chem., DOI: 10.1002/anie.201507796) have demonstrated a valuable synthetic strategy to access highly enantio- and diastereo-selective [4+2] cycloaddition or conjugate addition-protonation products from 5H-oxazol-4-ones and N-itaconimides starting substrates, using a L-tert-leucine-derived tertiary amine catalyst. By varying the reaction conditions — tuning the right chemical switches — enantio- and diastereo-enriched [4+2] or addition- protonation products could be achieved. Theoretical calculations accurately matched the observed product stereochemistry and indicated that the chemoselectivity was controlled by the relative thermodynamic stability of the [4+2] product, which transforms to the addition-protonation product once the reaction temperature is elevated.
Chemoselective switch in asymmetric organocatalysis
Most chemical reactions are straightforward and furnish only one set of products. However, a holy grail in synthesis is to design reactions that can be readily ‘tuned’ to deliver different product sets that can be selected according to the reaction conditions, reagents, catalysts and even post purification strategies. This task is even more challenging when enantio- and diastereo- discrimination is required. Collaborative work by researchers from Henan University and Australian National University (Zhu, B.; Lee, R.; Li, J.; Ye, X.; Hong, S.-N.; Qiu, S.; Coote, M.L.; Jiang, Z., Angew. Chem., DOI: 10.1002/anie.201507796) have demonstrated a valuable synthetic strategy to access highly enantio- and diastereo-selective [4+2] cycloaddition or conjugate addition-protonation products from 5H-oxazol-4-ones and N-itaconimides starting substrates, using a L-tert-leucine-derived tertiary amine catalyst. By varying the reaction conditions — tuning the right chemical switches — enantio- and diastereo-enriched [4+2] or addition- protonation products could be achieved. Theoretical calculations accurately matched the observed product stereochemistry and indicated that the chemoselectivity was controlled by the relative thermodynamic stability of the [4+2] product, which transforms to the addition-protonation product once the reaction temperature is elevated.
Zhu, B.; Lee, R.; Li, J.; Ye, X.; Hong, S.-N.; Qiu, S.; Coote, M.L.; Jiang, Z., Turning Chemoselective Switch in Asymmetric Organocatalysis of 5H-oxazol-4-ones and N-Itaconimides towards Tandem Conjugate Addition−Protonation or [4+2] Cycloaddition Angew. Chem., (2016) 55, 1299-1303.http://dx.doi.org/10.1002/anie.201507796
February 2016
ANU Reporter
 Supercharging Efficiency
Solar panels adorn the rooftops of more than one million Australian homes and the industry is booming. Yet most commercial solar cells are often relatively inefficient.
Many solar panels only convert up to 15 per cent of the sunlight received into electricity but ANU scientists are working on improving this rate.
Research by Professor Michelle Coote and a team of eight scientists from the ANU Research School of Chemistry has led to the doubling of the efficiency of solar cells using the National Computational Infrastructure (NCI) at ANU.
...
Read More
Supercharging Efficiency
Solar panels adorn the rooftops of more than one million Australian homes and the industry is booming. Yet most commercial solar cells are often relatively inefficient.
Many solar panels only convert up to 15 per cent of the sunlight received into electricity but ANU scientists are working on improving this rate.
Research by Professor Michelle Coote and a team of eight scientists from the ANU Research School of Chemistry has led to the doubling of the efficiency of solar cells using the National Computational Infrastructure (NCI) at ANU.
...
Read More
Gryn'ova G., Barakat J.M., Blinco J.P., Bottle S.E., Coote M.L., Computational Design of Cyclic Nitroxides as Efficient Redox Mediators for Dye-Sensitized Solar Cells Chem. Eur. J. (2012), 18, 7582-7593. http://dx.doi.org/10.1002/chem.201103598
October 2015
Chemistry in Australia
 Experimental Demonstration of pH-Dependent Electrostatic Catalysis of Radical Reactions
It is normally assumed that the ability to use electric fields to accelerate chemical reactions is limited to redox processes occurring at electrode surfaces. However, in theory, electric fields should be able to catalyse non-electrochemical reactions by electrostatically stabilising high-energy charge-separated resonance contributors to the transition state. In practice, the challenge is orienting the field relative to the reaction centre. One solution is to use charged functional groups within the substrate, auxiliary, or catalyst whose electric field is relatively localised but can be precisely aligned with respect to the reaction centre. If the charged functional group is an acid/base group, this approach has the added advantage of incorporating a pH switch for the catalytic effects. Researchers from the Australian National University have now provided a proof-of-concept of pH-dependent electrostatic catalysis by demonstrating a pH switch of up to two orders of magnitude on the rate and equilibrium constant for hydrogen atom transfer between a hydroxyl amine bearing a non-conjugated carboxylic acid and a reference profluorescent nitroxide radical (Klinska M., Smith L.M., Gryn'ova G., Banwell M.G., Coote M.L., Chem. Sci. 2015, doi:10.1039/C5SC01307K). Deprotonation of the carboxylic acid stabilises the resulting nitroxide radical anion, favouring the forward reaction. This ability to manipulate nitroxide stability via a simple pH switch opens the way to the possible use of this effect in synthetic and polymerisation processes.
Experimental Demonstration of pH-Dependent Electrostatic Catalysis of Radical Reactions
It is normally assumed that the ability to use electric fields to accelerate chemical reactions is limited to redox processes occurring at electrode surfaces. However, in theory, electric fields should be able to catalyse non-electrochemical reactions by electrostatically stabilising high-energy charge-separated resonance contributors to the transition state. In practice, the challenge is orienting the field relative to the reaction centre. One solution is to use charged functional groups within the substrate, auxiliary, or catalyst whose electric field is relatively localised but can be precisely aligned with respect to the reaction centre. If the charged functional group is an acid/base group, this approach has the added advantage of incorporating a pH switch for the catalytic effects. Researchers from the Australian National University have now provided a proof-of-concept of pH-dependent electrostatic catalysis by demonstrating a pH switch of up to two orders of magnitude on the rate and equilibrium constant for hydrogen atom transfer between a hydroxyl amine bearing a non-conjugated carboxylic acid and a reference profluorescent nitroxide radical (Klinska M., Smith L.M., Gryn'ova G., Banwell M.G., Coote M.L., Chem. Sci. 2015, doi:10.1039/C5SC01307K). Deprotonation of the carboxylic acid stabilises the resulting nitroxide radical anion, favouring the forward reaction. This ability to manipulate nitroxide stability via a simple pH switch opens the way to the possible use of this effect in synthetic and polymerisation processes.
Klinska, M.; Smith, L. M.; Gryn’ova, G.; Banwell, M. G.; Coote, M. L., Experimental Demonstration of pH-Dependent Electrostatic Catalysis of Radical Reactions Chem. Sci., (2015) 6, 5623–5627http://dx.doi.org/10.1039/C5SC01307K
April 2015
IUPAC-Solvay International Award for Young Chemists – 2015
 Ganna Gryn'ova wins one of the five IUPAC-Solvay International Award for Young Chemists, for her Ph.D. thesis work entitled “Understanding and Manipulating The Reactivity of Nitroxides and Other Stable Free Radicals”.
Ganna Gryn'ova wins one of the five IUPAC-Solvay International Award for Young Chemists, for her Ph.D. thesis work entitled “Understanding and Manipulating The Reactivity of Nitroxides and Other Stable Free Radicals”.
February 2015
Chemistry World
 Colourless spiropyrans undergo ring opening to form brightly coloured merocyanines on exposure to UV light. Merocyanines are thermally unstable and relax back to the colourless spiropyrans over time. The merocyanines designed by Simone Ciampi, from the University of Wollongong, Australia, and his colleagues contain a catechol group that can form intramolecular hydrogen bonds, which stabilises the open form and slows down discolouration. However, polar solvents can out-compete intramolecular hydrogen bond formation, and speed up discolouration. In this way, Ciampi’s team were able to visualise the hydrogen bonding character of solvents by adding their dye and observing the rate at which it discoloured.
Colourless spiropyrans undergo ring opening to form brightly coloured merocyanines on exposure to UV light. Merocyanines are thermally unstable and relax back to the colourless spiropyrans over time. The merocyanines designed by Simone Ciampi, from the University of Wollongong, Australia, and his colleagues contain a catechol group that can form intramolecular hydrogen bonds, which stabilises the open form and slows down discolouration. However, polar solvents can out-compete intramolecular hydrogen bond formation, and speed up discolouration. In this way, Ciampi’s team were able to visualise the hydrogen bonding character of solvents by adding their dye and observing the rate at which it discoloured.
Ciampi, Eggers, Haworth, Darwish, Wagner, Coote, Wallace, Raston, Chem. Commun., (2015) Advance Article.http://dx.doi.org/10.1039/C4CC09857A
August 2014
International Innovation
 Computerised chemicals.
Quantum chemist Professor Michelle Coote explains the unique opportunities offered by her field of science, describing her own work towards developing efficient computer models for investigating chemical reactions of industrial relevance.
Read more...
Computerised chemicals.
Quantum chemist Professor Michelle Coote explains the unique opportunities offered by her field of science, describing her own work towards developing efficient computer models for investigating chemical reactions of industrial relevance.
Read more...
July 2014
RSC Molecule of the Month
 Li+ Catalysed Polymerization.
Pulsed Laser Polymerization studies have demonstrated that LiNTf2 catalyzes propagation in the radical polymerization of methyl methacrylate. Theoretical calculations reveal that catalysis arises from monomer activation, occurring at the expense of tacticity regulation. Propagation catalysts can be used to improve molecular weight control and suppress chain-branching.
Noble, Smith, Coote Polym. Chem., 2014, DOI 10.1039/C4PY00190G
Read more...
Li+ Catalysed Polymerization.
Pulsed Laser Polymerization studies have demonstrated that LiNTf2 catalyzes propagation in the radical polymerization of methyl methacrylate. Theoretical calculations reveal that catalysis arises from monomer activation, occurring at the expense of tacticity regulation. Propagation catalysts can be used to improve molecular weight control and suppress chain-branching.
Noble, Smith, Coote Polym. Chem., 2014, DOI 10.1039/C4PY00190G
Read more...
May 2014
Science at the Shine Dome 2014
Computer-Aided Chemical Design - The Future of Chemistry? Michelle's New Fellows Presentation
March 2014
Michelle Coote elected to the Fellowship of the Australian Academy of Science
 Academy welcomes science leaders to Fellowship
The Australian Academy of Science today announced the election of 21 leaders in Australian science to its Fellowship, including those working on how galaxies and stars are formed, epilepsy prevention and quantum teleportation. The new Fellows have been elected for their outstanding contributions to and application of scientific research. Academy President Suzanne Cory congratulated all of the new Fellows for their stellar achievements and contributions to advancing the sum of human knowledge. Every year the Academy honours the work of Australia's leading scientists with election to its Fellowship, which now numbers 481. New Fellows will be formally admitted to the Academy at its annual flagship event, Science at the Shine Dome, in Canberra this May, where they will make short presentations about their work.
Read more...
Academy welcomes science leaders to Fellowship
The Australian Academy of Science today announced the election of 21 leaders in Australian science to its Fellowship, including those working on how galaxies and stars are formed, epilepsy prevention and quantum teleportation. The new Fellows have been elected for their outstanding contributions to and application of scientific research. Academy President Suzanne Cory congratulated all of the new Fellows for their stellar achievements and contributions to advancing the sum of human knowledge. Every year the Academy honours the work of Australia's leading scientists with election to its Fellowship, which now numbers 481. New Fellows will be formally admitted to the Academy at its annual flagship event, Science at the Shine Dome, in Canberra this May, where they will make short presentations about their work.
Read more...
 Top thinkers entered into Australian Academy of Science
A self-described "discovery-junkie" with a passion for biodiversity will take his place among Australia's scientific elite this May. Of 21 researchers who have been elected fellows of the Australian Academy of Science, more than a third are based in Canberra. Craig Moritz and six of his colleagues from the Australian National University will be among the group, along with Elizabeth Jean Finnegan who works in the plant industry division at CSIRO Black Mountain. A further three researchers from interstate institutions make use of the National Computational Infrastructure facilities at the ANU. The academy each year elects some of Australia's best scientists to its fellowship, which now stands at 481.
Read more...
Top thinkers entered into Australian Academy of Science
A self-described "discovery-junkie" with a passion for biodiversity will take his place among Australia's scientific elite this May. Of 21 researchers who have been elected fellows of the Australian Academy of Science, more than a third are based in Canberra. Craig Moritz and six of his colleagues from the Australian National University will be among the group, along with Elizabeth Jean Finnegan who works in the plant industry division at CSIRO Black Mountain. A further three researchers from interstate institutions make use of the National Computational Infrastructure facilities at the ANU. The academy each year elects some of Australia's best scientists to its fellowship, which now stands at 481.
Read more...
Dec 2013
ARC Centre of Excellence for Electromaterials Science Announced
 Coote group joins ACES.
The ARC Centre of Excellence for Electromaterials Science (ACES) has received $25million as a new ARC Centre of Excellence to further develop their work on smart nano-materials, to now create 3D devices with advanced capabilities over their 2D counterparts. The resulting technology breakthroughs will have a direct impact on existing industries for batteries, solar cells and medical implants and will provide opportunities for the development of new manufacturing capabilities.
Read more...
Coote group joins ACES.
The ARC Centre of Excellence for Electromaterials Science (ACES) has received $25million as a new ARC Centre of Excellence to further develop their work on smart nano-materials, to now create 3D devices with advanced capabilities over their 2D counterparts. The resulting technology breakthroughs will have a direct impact on existing industries for batteries, solar cells and medical implants and will provide opportunities for the development of new manufacturing capabilities.
Read more...
Chemistry in Australia
 Non-directional polar effects on radical stability.
Polar effects on radical stability are traditionally attributed either to resonance effects or to dipole effects. The former involves donor-acceptor interactions between specific functional groups that are conjugated or hyperconjugated with one another; the latter involves through space electrostatic interactions between charged (or partially charge-separated) functional groups. Both types of effects are strongly directional. However, researchers from the Australian National University have now discovered a third type of polar effect that is in principle non-directional and requires no conjugation or permanent dipoles (G. Gryn'ova, M. L. Coote J. Am. Chem. Soc. 2013, 135, 15392).
http://pubs.acs.org/doi/abs/10.1021/ja404279f
Non-directional polar effects on radical stability.
Polar effects on radical stability are traditionally attributed either to resonance effects or to dipole effects. The former involves donor-acceptor interactions between specific functional groups that are conjugated or hyperconjugated with one another; the latter involves through space electrostatic interactions between charged (or partially charge-separated) functional groups. Both types of effects are strongly directional. However, researchers from the Australian National University have now discovered a third type of polar effect that is in principle non-directional and requires no conjugation or permanent dipoles (G. Gryn'ova, M. L. Coote J. Am. Chem. Soc. 2013, 135, 15392).
http://pubs.acs.org/doi/abs/10.1021/ja404279f
Read more...
RSC Molecule of the Month
 Non-directional Polar Effects
Polar effects on radical stability are traditionally attributed either to resonance or dipole interactions, both of which are directional. However, Anya Gryn'ova and Michelle Coote have now discovered a third type of polar effect that is in principle non-directional and requires no conjugation or permanent dipoles. When a localised anion is placed in the vicinity of a delocalised radical, the radical is strongly stabilized compared with its corresponding non-radical derivatives due to its enhanced polarizability. The arising polar effects are surprisingly large and long range, and are likely to be useful in synthesis and harnessed in enzyme catalysis. (Gryn'ova G., Coote M.L. J. Am. Chem. Soc. (2013), 135, 15392-15403.
http://pubs.acs.org/doi/abs/10.1021/ja404279f
Non-directional Polar Effects
Polar effects on radical stability are traditionally attributed either to resonance or dipole interactions, both of which are directional. However, Anya Gryn'ova and Michelle Coote have now discovered a third type of polar effect that is in principle non-directional and requires no conjugation or permanent dipoles. When a localised anion is placed in the vicinity of a delocalised radical, the radical is strongly stabilized compared with its corresponding non-radical derivatives due to its enhanced polarizability. The arising polar effects are surprisingly large and long range, and are likely to be useful in synthesis and harnessed in enzyme catalysis. (Gryn'ova G., Coote M.L. J. Am. Chem. Soc. (2013), 135, 15392-15403.
http://pubs.acs.org/doi/abs/10.1021/ja404279f
Read more...
Oct 2013
NYSF Annual Report
 Alumni Testimonials: Professor Michelle Coote.
Michelle Coote attended the NYSF (then called National Science Summer School (NSSS) in January 1990. She had always had an interest in science and particularly astronomy, and was in the Galileo group at the NYSF.
Read more...
Alumni Testimonials: Professor Michelle Coote.
Michelle Coote attended the NYSF (then called National Science Summer School (NSSS) in January 1990. She had always had an interest in science and particularly astronomy, and was in the Galileo group at the NYSF.
Read more...
Sep 2013
RSC Molecule of the Month
 Ozone and Goliath.
Quantum chemical calculations reveal that ozone (~100 ppbv) is capable of instigating a series of degradative processes in pol(methyl methacrylate). The crux lies in the the self decomposition of 1,2,4-trioxolane, which undergoes a spin intersystem crossing process through the Minimum Energy Crossing Point (MECP) and O-O bond cleavage to unravel a triplet biradical intermediate that facilitates auto-degradation of the polymer chain. This process which is 33 times more efficient than normal degradation in air suggests competitive damage by ozone to acrylate despite its very low concentration. (Lee R., Coote M.L., Phys. Chem. Chem. Phys. 2013, 15, 16428-16431).
http://dx.doi.org/10.1039/C3CP52863D
Ozone and Goliath.
Quantum chemical calculations reveal that ozone (~100 ppbv) is capable of instigating a series of degradative processes in pol(methyl methacrylate). The crux lies in the the self decomposition of 1,2,4-trioxolane, which undergoes a spin intersystem crossing process through the Minimum Energy Crossing Point (MECP) and O-O bond cleavage to unravel a triplet biradical intermediate that facilitates auto-degradation of the polymer chain. This process which is 33 times more efficient than normal degradation in air suggests competitive damage by ozone to acrylate despite its very low concentration. (Lee R., Coote M.L., Phys. Chem. Chem. Phys. 2013, 15, 16428-16431).
http://dx.doi.org/10.1039/C3CP52863D
Read more...
Chemistry in Australia
 Harnessing entropy to heal polymers.
Self-healing polymers are based on dynamically bonding functional groups that allow the material to undergo reversible de-bonding in response to a pre-selected trigger such as heat, light or pH. Currently, the method for tuning the de-bonding point of such materials is to design the reversibly bonding functional groups (i.e. the chemistry), which essentially requires new monomers to be synthesised each time an adjustment of the de-bonding point is desired. However, scientists from the Australian National University, the Karlsruhe Institute of Technology, the University of Dresden and Evonik Industries report that the de-bonding temperature of a polymer can also be tuned by changing the chain length of the polymer building blocks, thus altering the entropy released on debonding (Guimard N.K., Ho J., Brandt J., Lin C.Y., Namazian M., Mueller J.O., Oehlenschlager K.K., Hilf S., Lederer A., Schmidt F.G., Coote M.L., Barner-Kowollik C. Chem. Sci. 2013, 4, 2752-59).
Read more...
Harnessing entropy to heal polymers.
Self-healing polymers are based on dynamically bonding functional groups that allow the material to undergo reversible de-bonding in response to a pre-selected trigger such as heat, light or pH. Currently, the method for tuning the de-bonding point of such materials is to design the reversibly bonding functional groups (i.e. the chemistry), which essentially requires new monomers to be synthesised each time an adjustment of the de-bonding point is desired. However, scientists from the Australian National University, the Karlsruhe Institute of Technology, the University of Dresden and Evonik Industries report that the de-bonding temperature of a polymer can also be tuned by changing the chain length of the polymer building blocks, thus altering the entropy released on debonding (Guimard N.K., Ho J., Brandt J., Lin C.Y., Namazian M., Mueller J.O., Oehlenschlager K.K., Hilf S., Lederer A., Schmidt F.G., Coote M.L., Barner-Kowollik C. Chem. Sci. 2013, 4, 2752-59).
Read more...
Aug 2013
Materials Views
 Spotlight on Polymer Chemistry.
A polymer's tacticity can affect important physical properties such as melting point, glass transition temperature, and mechanical strength. Radical polymerization is an inexpensive route to producing high yield polymers, but it has no control over tacticity. Michelle L. Coote and Leese M. Smith aim to provide benchmark values for tacticity of polymers produced by radical polymerization of styrene over a variable range of solvents and temperatures.
Read more...
Spotlight on Polymer Chemistry.
A polymer's tacticity can affect important physical properties such as melting point, glass transition temperature, and mechanical strength. Radical polymerization is an inexpensive route to producing high yield polymers, but it has no control over tacticity. Michelle L. Coote and Leese M. Smith aim to provide benchmark values for tacticity of polymers produced by radical polymerization of styrene over a variable range of solvents and temperatures.
Read more...
Chemistry in Australia
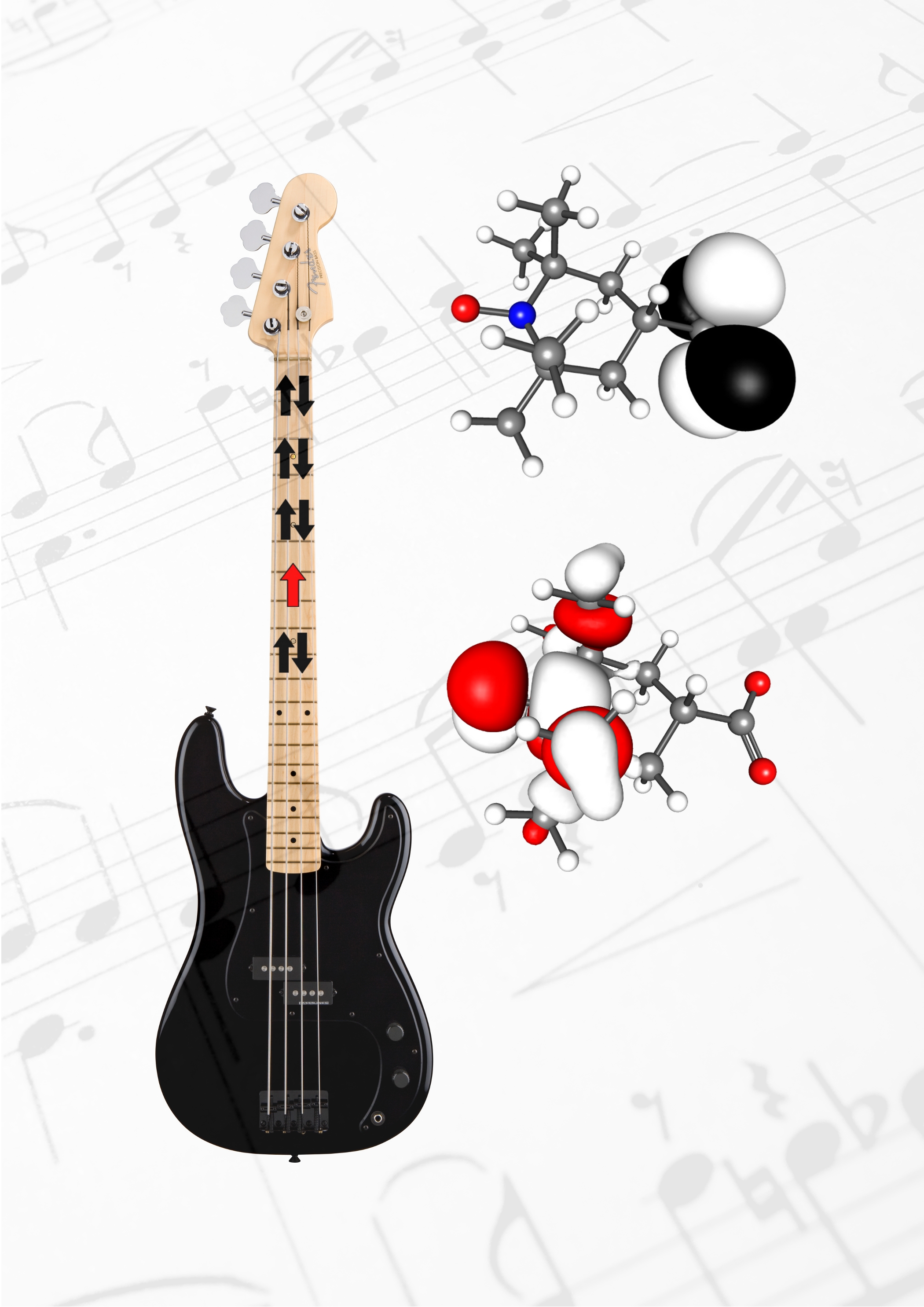 Radical orbital switching.
A recent discovery made by researchers from the Australian National University and University of Wollongong appears to challenge several cornerstones of chemical reactivity (Gryn'ova G., Marshall D.L., Blanksby S.J., Coote M.L., Nature Chem. 2013, 5, 474-81). According to the aufbau principle, the unpaired electron of a free radical should occupy the highest-energy orbital. However, the authors show that a broad range of distonic radical anions display so-called orbital energy-level conversion, in which their singly-occupied orbital is energetically lower than the doubly-occupied orbital(s) of the anionic functional group.
Read more...
Radical orbital switching.
A recent discovery made by researchers from the Australian National University and University of Wollongong appears to challenge several cornerstones of chemical reactivity (Gryn'ova G., Marshall D.L., Blanksby S.J., Coote M.L., Nature Chem. 2013, 5, 474-81). According to the aufbau principle, the unpaired electron of a free radical should occupy the highest-energy orbital. However, the authors show that a broad range of distonic radical anions display so-called orbital energy-level conversion, in which their singly-occupied orbital is energetically lower than the doubly-occupied orbital(s) of the anionic functional group.
Read more...
Jul 2013
RSC News, mechanism of the month
 Gryn'ova G., Marshall D.L., Blanksby S.J. and Coote M.L.
Switching Radical Stability By pH-Induced Orbital Conversion
Nature Chem.(2013), 5, 474-481.
http://dx.doi.org/10.1038/NCHEM.1625
Gryn'ova G., Marshall D.L., Blanksby S.J. and Coote M.L.
Switching Radical Stability By pH-Induced Orbital Conversion
Nature Chem.(2013), 5, 474-481.
http://dx.doi.org/10.1038/NCHEM.1625
Read more...
Group of Eight
 Michelle Coote highlighted in Group of Eight booklet showcasing the value of the ARC Future Fellowships scheme. Plastics are all around us, but we rarely spare a thought for the way they've revolutionised our lives. When we do, it's concern about how much plastic is going into landfill each year. Professor Michelle Coote at The Australian National University (ANU) is researching ways to make longer-lasting and more biodegradable plastics, from the polyesters used for coating Colorbond steel, to the polyvinyl chloride (PVC) used to insulate electrical cables.
Read more...
Michelle Coote highlighted in Group of Eight booklet showcasing the value of the ARC Future Fellowships scheme. Plastics are all around us, but we rarely spare a thought for the way they've revolutionised our lives. When we do, it's concern about how much plastic is going into landfill each year. Professor Michelle Coote at The Australian National University (ANU) is researching ways to make longer-lasting and more biodegradable plastics, from the polyesters used for coating Colorbond steel, to the polyvinyl chloride (PVC) used to insulate electrical cables.
Read more...
May 2013
Nature Chemistry News and Views
 Reactive intermediates: Radicals with multiple personalities
A combined theoretical and experimental approach has revealed that radicals can be significantly stabilized by the presence of a remote anionic site in the same molecule. This finding has implications for understanding and potentially controlling the reactivity of these important reactive intermediates.Read more...
Reactive intermediates: Radicals with multiple personalities
A combined theoretical and experimental approach has revealed that radicals can be significantly stabilized by the presence of a remote anionic site in the same molecule. This finding has implications for understanding and potentially controlling the reactivity of these important reactive intermediates.Read more...
Chemical Science Blog
 Controlling the bonding/debonding of polymer systems Controlling polymer debonding/rebonding properties using responsive materials is an exciting emerging area of chemistry and it is widely accepted that control of these properties can be achieved by engineering the functional end-groups responsible for monomer dynamic bonding.
Read more...
Controlling the bonding/debonding of polymer systems Controlling polymer debonding/rebonding properties using responsive materials is an exciting emerging area of chemistry and it is widely accepted that control of these properties can be achieved by engineering the functional end-groups responsible for monomer dynamic bonding.
Read more...
Mar 2013
SCM News
 pKa calculations by Junming Ho well received. During his PhD, Junming Ho focused on how to best predict pKa values with theoretical methods. His work was very well received, his PhD thesis "Predicting pKa: Theory and Applications" received both the 2012 RACI Cornforth Medal and the Director's Prize for Best Chemistry PhD Thesis..
Read more...
pKa calculations by Junming Ho well received. During his PhD, Junming Ho focused on how to best predict pKa values with theoretical methods. His work was very well received, his PhD thesis "Predicting pKa: Theory and Applications" received both the 2012 RACI Cornforth Medal and the Director's Prize for Best Chemistry PhD Thesis..
Read more...
Oct 2012
Chemistry in Australia
 Extraordinary efficiency of amine antioxidants explained. Hindered amine light stabilisers (HALSs) are remarkably effective radical-trapping antioxidants; however, the precise mechanism of their protective action has long been debated.
Read more...
Extraordinary efficiency of amine antioxidants explained. Hindered amine light stabilisers (HALSs) are remarkably effective radical-trapping antioxidants; however, the precise mechanism of their protective action has long been debated.
Read more...
RSC News, molecule of the month
 Heinrich N., Willis A.C., Cade I.A., Ho J., Coote M.L., and Banwell M.G.,
Reversible Cyclopropane Ring-Cleavage Reactions within Etheno-Bridged [5.3.1]Propelladiene Frameworks Leading to Aza- and Oxa- [5.6.5.6]Fenestratetraenes
Chem. Eur. J., (2012), 18, 13585-13588.
http://dx.doi.org/10.1002/chem.201202903
Heinrich N., Willis A.C., Cade I.A., Ho J., Coote M.L., and Banwell M.G.,
Reversible Cyclopropane Ring-Cleavage Reactions within Etheno-Bridged [5.3.1]Propelladiene Frameworks Leading to Aza- and Oxa- [5.6.5.6]Fenestratetraenes
Chem. Eur. J., (2012), 18, 13585-13588.
http://dx.doi.org/10.1002/chem.201202903
Read more...
Sep 2012
JACS Image Challenge
 Gryn'ova G., Ingold K.U., Coote M.L.,
New Insights into the Mechanism of Amine/Nitroxide Cycling during the Hindered Amine Light Stabilizer Inhibited Oxidative Degradation of Polymers
J. Am. Chem. Soc., (2012), 134, 12979-12988.
http://dx.doi.org/10.1021/ja3006379
Gryn'ova G., Ingold K.U., Coote M.L.,
New Insights into the Mechanism of Amine/Nitroxide Cycling during the Hindered Amine Light Stabilizer Inhibited Oxidative Degradation of Polymers
J. Am. Chem. Soc., (2012), 134, 12979-12988.
http://dx.doi.org/10.1021/ja3006379
Take the challenge today!
RSC News, mechanism of the month
 Gryn'ova G., Ingold K.U., Coote M.L.,
New Insights into the Mechanism of Amine/Nitroxide Cycling during the Hindered Amine Light Stabilizer Inhibited Oxidative Degradation of Polymers
J. Am. Chem. Soc., (2012), 134, 12979-12988.
http://dx.doi.org/10.1021/ja3006379
Gryn'ova G., Ingold K.U., Coote M.L.,
New Insights into the Mechanism of Amine/Nitroxide Cycling during the Hindered Amine Light Stabilizer Inhibited Oxidative Degradation of Polymers
J. Am. Chem. Soc., (2012), 134, 12979-12988.
http://dx.doi.org/10.1021/ja3006379
Read more...
Aug 2012
JACS Spotlight
 NEW EXPLANATION FOR PRESERVATION OF POLYMERS BY AMINE ANTIOXIDANTS
NEW EXPLANATION FOR PRESERVATION OF POLYMERS BY AMINE ANTIOXIDANTS
Antioxidants are crucial to life and have commercial uses such as preventing disruptive substances from forming in gasoline and protecting plastics from degradation. Michelle Coote and co-workers have uncovered new information about the protective action of hindered amine light stabilizers (HALSs), a type of amine-based radical-trapping antioxidant (DOI: 10.1021/ja3006379). Phenols and amines are the two main classes of radical-trapping antioxidants (RTAs), and while the mechanism by which phenol RTAs act is well known, the reaction pathways of amine RTAs are far less clear.
Read more...
May 2012
Science Wise
 How chemists are increasing the longevity of plastics
How chemists are increasing the longevity of plastics
Whenever you walk along the seashore, you're bound to see some sort of plastic littering the beach and perhaps you wonder will it ever decompose? Then you go home and see your garden hose cracked and falling to pieces and you wonder why that plastic can't be made to last as long as the litter on the beach! From an industrial point of view, longevity of polymers is something that it would be highly desirable
Read more...
Jan 2012
Canberra Times
 Professor Urges Supermarket Bag Boycott
Professor Urges Supermarket Bag Boycott
A polymer expert has urged Canberrans to boycott plastic bags sold at supermarket checkouts and instead buy compostable bags and bin liners. Professor of chemistry at the Australian National University Michelle Coote said the bags sold by Coles and Woolworths in response to an ACT Government ban on plastic bags thinner than 35 microns would never really breakdown, but rather just break into smaller and smaller pieces.
Read more...
Oct 2011
JACS Image Challenge
 Ho J., Easton C. J., Coote M.L.
The Distal Effect of Electron-Withdrawing Groups and Hydrogen Bonding on the Stability of Peptide Enolates.
J. Am. Chem. Soc. (2010), 132, 5515-5521.
http://dx.doi.org/10.1021/ja100996z
Ho J., Easton C. J., Coote M.L.
The Distal Effect of Electron-Withdrawing Groups and Hydrogen Bonding on the Stability of Peptide Enolates.
J. Am. Chem. Soc. (2010), 132, 5515-5521.
http://dx.doi.org/10.1021/ja100996z
Take the challenge today!
Sep 2011
JACS Image Challenge
 Isse A. A., Gennaro A., Lin C. Y., Hodgson J. L., Coote M.L., Tamaz G.
Mechanism of Carbon-Halogen Bond Reductive Cleavage in Activated Alkyl Amine Halide Initiators Relevant to Living Radical Polymerization" Theoretical and Expeirmental Study.
J. Am. Chem. Soc. (2011), 113, 6254-6264.
http://dx.doi.org/10.1021/ja110538b
Isse A. A., Gennaro A., Lin C. Y., Hodgson J. L., Coote M.L., Tamaz G.
Mechanism of Carbon-Halogen Bond Reductive Cleavage in Activated Alkyl Amine Halide Initiators Relevant to Living Radical Polymerization" Theoretical and Expeirmental Study.
J. Am. Chem. Soc. (2011), 113, 6254-6264.
http://dx.doi.org/10.1021/ja110538b
Take the challenge today!
Jul 2011
RSC News, molecule of the month
 Ho J., Coote M.L., Easton C. J.
Validation of the Distal Effect of Electron-withdrawing Groups on the Stability of Peptide Enolates and Its Exploitation in the Controlled Stereochemical Inversion of Amino Acid Derivatives
J. Org. Chem. (2011), 76, 5907-5914.
http://dx.doi.org/10.1021/jo200994z
Ho J., Coote M.L., Easton C. J.
Validation of the Distal Effect of Electron-withdrawing Groups on the Stability of Peptide Enolates and Its Exploitation in the Controlled Stereochemical Inversion of Amino Acid Derivatives
J. Org. Chem. (2011), 76, 5907-5914.
http://dx.doi.org/10.1021/jo200994z
Read more...
May 2011
Materials Views
 A mechanistic tug-of-war: Initiation via ISET or OSET
by Marie-Claire Hermant published: 2011-05-06
A mechanistic tug-of-war: Initiation via ISET or OSET
by Marie-Claire Hermant published: 2011-05-06
The advent of controlled/ living radical polymerization (LRP) techniques, including atom transfer radical polymerization (ATRP) amongst others, has enabled polymer and material scientists to have very fine control over polymer molecular weights, compositions and structures. The mechanistic Read more...
Apr 2011
Gardening Australia
 Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Read more...
Feb 2011
Plastics in Packaging
 Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Read more...
Feb 2011
ACS magazine, Chemical and Engineering News.
 Mechanistic Study Boosts Knowledge of Radical Polymerization
Mechanistic Study Boosts Knowledge of Radical Polymerization
A.A. Isse, A. Gennaro, C.Y. Lin, J.L. Hodgson, M.L. Coote, and T. Guliashvili
Mechanism of Carbon-Halogen Bond Reductive Cleavage in Activated Alkyl Halide Initiators Relevant to Living Radical Polymerization: Theoretical and Experimental Study, J. Am. Chem. Soc. (2011), 133, 6254-6264.
http://dx.doi.org/10.1021/ja110538b
Read more...
Jan 2011
RSC News, molecule of the month
 Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Read more...
Dec 2010
Chemistry in Australia
 Living Luminaries of Australian Chemistry: Michelle Coote
Living Luminaries of Australian Chemistry: Michelle Coote
'If I could start again I would have learnt a lot more biochemistry - I think there are a lot of interesting problems still to be solved at the interface of chemistry and biology ...' -- Michelle Coote
Read more...
Dec 2010
Canberra Times
 ANU graduate honours sustainability
ANU graduate honours sustainability
http://www.canberratimes.com.au/news/local/news/general/anu-graduate-honours-sustainability/2023029.aspx
While undertaking her degree at the Australian National University, Zemma Holmes-Story ventured to America to complete a sustainability internship at Yale University. The trip sparked a number of ideas, one being the sustainable mug program she implemented at the university in Canberra.
Read more...
Nov 2010
Science Alert
 Making stronger clothes pegs
Making stronger clothes pegs
Gryn'ova G., Hodgson J.L., Coote M.L.
Revising the mechanism of polymer autooxidation.
Org. Biomol. Chem. (2011), 9(2), 480-490.
http://dx.doi.org/10.1039/C0OB00596G
Read more...
May 2010
RSC News, molecule of the month
 Ho J., Easton C.J., Coote M.L. The distal effect of electron-withdrawing groups and hydrogen bonding on the stability of peptide enolates. J. Am. Chem. Soc. (2010), 132(15), 5515 - 5521. http://dx.doi.org/10.1021/ja100996z
Ho J., Easton C.J., Coote M.L. The distal effect of electron-withdrawing groups and hydrogen bonding on the stability of peptide enolates. J. Am. Chem. Soc. (2010), 132(15), 5515 - 5521. http://dx.doi.org/10.1021/ja100996z
Read more...
Nov 2009
RSC News, molecule of the month
 Lin CY, Coote ML, Gennaro A, Matyjaszewski K Ab initio evaluation of the thermodynamic and electrochemical properties of alkyl halides and radicals and their mechanistic implications for atom transfer radical polymerization. J. Am. Chem. Soc. (2008), 130(38), 12762-12774.
http://dx.doi.org/10.1021/ja8038823
Lin CY, Coote ML, Gennaro A, Matyjaszewski K Ab initio evaluation of the thermodynamic and electrochemical properties of alkyl halides and radicals and their mechanistic implications for atom transfer radical polymerization. J. Am. Chem. Soc. (2008), 130(38), 12762-12774.
http://dx.doi.org/10.1021/ja8038823
Read more...
Apr 2009
Canberra Times
 Chemistry connections
Chemistry connections
Supercomputers are offering a window into the minuscule world of chemical reactions. What scientists see can help them better understand the mechanics of molecules - how they interact and how they make reactions speed up or slow down. Read more...
May 2008
Science Wise
 Beyond the test tube -- Supercomputer quantum chemistry in the twenty-first century
Beyond the test tube -- Supercomputer quantum chemistry in the twenty-first century
Chemistry is the study of whether or not substances react and what they produce if they do. Chemists know how different chemicals interact from their experiments, but what if they could predict how they react by simply running a computer program? Read more...
May 2008
Science Education News
 Computerational Chemistry: The State of the Art
Computerational Chemistry: The State of the Art
The reactiviy of chemical compounds is governed by energies: how much energy is released or required for a reaction to take place, and how large an energy barrier exists between the reactants and the products. These energies can be determined using computers, and are used to predict how fast a reaction is, and whether it will occur or not. However, Read more...
Apr 2008
Science Alert
 Supercomputer creates drugs faster
Supercomputer creates drugs faster
The development of new drugs has been given a high-tech speed boost by chemists at the Australian National University using a supercomputer. The ability for drug molecules to donate or accept electrons, Read more...
Apr 2008
Bio Technology News
 ANU modelling may cut target compound work
ANU modelling may cut target compound work
Optmising small molecule drug development may be easier to do inside a computer than a laboratory, according to Australian National University scientists working at the ARC Centre of Excellence for Free Radical Chemistry and Biotechnology. Read more...