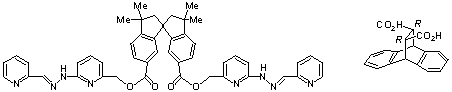
Inorganic Chemistry
 Coordination chemistry has merged with organic and
organometallic chemistry and with catalysis with the result that there
are now modifications available for nearly every standard reaction for
converting achiral organic precursors into chiral products. Together
with modern purification techniques, this has allowed the preparation
– in a single step – of compounds in >98% enantiomeric
purity for many reaction types. Work in this group is concerned with
the synthesis of new types of chiral ligands, especially
enantiomerically pure phosphines and arsines, for use as probes of
inorganic stereochemistry, rearrangements in metal complexes, and as
auxiliaries for asymmetric synthesis.
Coordination chemistry has merged with organic and
organometallic chemistry and with catalysis with the result that there
are now modifications available for nearly every standard reaction for
converting achiral organic precursors into chiral products. Together
with modern purification techniques, this has allowed the preparation
– in a single step – of compounds in >98% enantiomeric
purity for many reaction types. Work in this group is concerned with
the synthesis of new types of chiral ligands, especially
enantiomerically pure phosphines and arsines, for use as probes of
inorganic stereochemistry, rearrangements in metal complexes, and as
auxiliaries for asymmetric synthesis.
Heather Kitto and Rebecca Warr received grants from the Australian–German Joint Research Cooperation Scheme to work as exchange students in the University of Leipzig for one month (August). They visited en route to Leipzig the University of Cambridge and on the way home the Technical University Munich, where they presented lectures on their work in Canberra. Professor Wild accompanied the students on a grant funded by the Alexander von Humboldt Foundation. He presented a lecture in the University of Cambridge and an invited lecture at the INTAS Workshop 2003 on Phosphines, Chirality and Catalysis, which was held between 18–20 August in the University of Leipzig. Four postgraduate students from the University of Leipzig (Martina Hanner, Ulrike Helmstedt, Thomas Hoecher, and Uwe Polster) worked in the group for 1–3 months each during 2003 on grants funded by the Deutzche Akademischer Austausch Dienst (DAAD). Professor Wild was elected in April 2003 as a Fellow of the Australian Academy of Science.
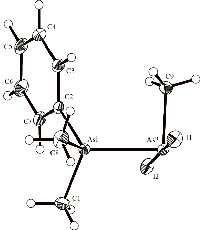
In connection with our investigations of tertiary phosphine and
arsine-stabilised arsenium salts, we have synthesised the adducts
PhMe2As•AsI2Me, PhMeEtAs•AsI2Me and
PhMe2As•AsI2Ph, which were first
isolated by Burrows and Turner (J. Chem. Soc.,1920,
117, 1373). The crystal structures of the three compounds have
been determined. The structure of PhMe2As
•AsI2Me is shown. In the
structure, the AsAsI angles and the AsAsC angle of the iodoarsine are
ca. 90°. Density functional theory calculations on the
three structures are in agreement with the experimental data.
(with S. Petrie, X. Zhou, and R. Stranger [Dept. Chemistry, ANU])
We have embarked on a program aimed at demonstrating that chiral metal complexes can be prepared by asymmetric synthesis – inorganic asymmetric synthesis. The approach being adopted is to transfer chiral information to a metal centre by means of an enantiomerically pure chiral auxiliary attached to appropriate chelating agents, as in (±)-1, with the auxiliary group subsequently being removed to leave the configurationally pure metal complex. Ligand (±)-1 diastereoselectively complexes zinc(II) to produce a dizinc(II) helicate; the S enantiomer of the ligand generates two metal centres of Δ configuration in the double-stranded helicate. Current work is concerned with the enantioselective synthesis of the analogous compound using the readily accessible chiral auxiliary (R)-2. (with R.J. Warr, A.C. Willis)
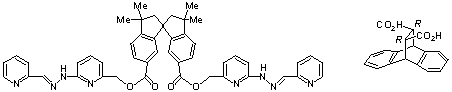
|
|
|
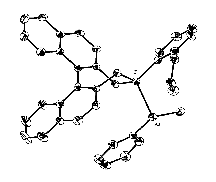 Previous work has shown that the reaction of iodoarsines with tertiary
phosphines in the presence of hexafluorophosphate ion gives adducts
of the type [R3P → AsR1R2]PF6,
which react with carbanionic nucleophiles (Nu–) to
give tertiary arsines (±)-AsR1R2Nu.
Recently, a binaphthyl-substituted phosphepine has been identified as
a very effective chiral auxiliary for the reaction, generating, via
the arsenium adduct shown, arsines in enantiomeric excesses of up to
77%. This result is now being submitted to further testing, to define
the conditions producing maximum stereoselectivity.
(with E.H. Krenske, K.A. Porter, A.C. Willis)
Previous work has shown that the reaction of iodoarsines with tertiary
phosphines in the presence of hexafluorophosphate ion gives adducts
of the type [R3P → AsR1R2]PF6,
which react with carbanionic nucleophiles (Nu–) to
give tertiary arsines (±)-AsR1R2Nu.
Recently, a binaphthyl-substituted phosphepine has been identified as
a very effective chiral auxiliary for the reaction, generating, via
the arsenium adduct shown, arsines in enantiomeric excesses of up to
77%. This result is now being submitted to further testing, to define
the conditions producing maximum stereoselectivity.
(with E.H. Krenske, K.A. Porter, A.C. Willis)
| Previous work in our laboratory has shown that (S,S)-tetraphos spontaneously self-assembles dinuclear metal helicates of the type (M)-[M2{(R,R)-tetraphos}2](PF6)2 upon reaction with univalent silver and gold salts. These complexes have a double α–helical or side-by-side parallel helix conformation depending on the twist of the central 10-membered ring containing the two metal ions, which has the chiral twist-boat–chair–boat conformation. The ligand (RC)-Me-tetraphos is currently being synthesised by a novel route involving the ring-opening of a chiral phosphirane. |
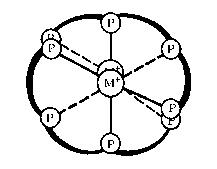 double α helix |
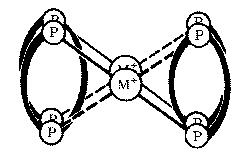 parallel helix |
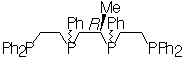
(RC)-Me-tetraphos