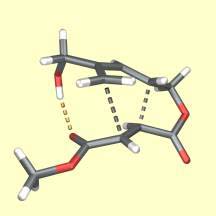
A hydrogen bond (bronze) improves the stereoselectivity of a Diels–Alder reaction.
Organic Chemistry
 Domino reactions are spectacular events in which many bonds
are made and broken in a single step. These reactions hold much
promise for achieving more efficient syntheses: a pressing need in
times of increasing production costs and the importance of protecting
the environment by reducing waste. Our research program involves the
design and implementation of sequences of cycloaddition reactions,
free radical reactions and transition metal-mediated reactions to
prepare polycyclic molecules with important biological
properties. This program also targets new ways to achieve molecular
recognition, complexation and catalysis. Overall, the primary goal is
to synthesise such complex molecules in a practical manner.
Domino reactions are spectacular events in which many bonds
are made and broken in a single step. These reactions hold much
promise for achieving more efficient syntheses: a pressing need in
times of increasing production costs and the importance of protecting
the environment by reducing waste. Our research program involves the
design and implementation of sequences of cycloaddition reactions,
free radical reactions and transition metal-mediated reactions to
prepare polycyclic molecules with important biological
properties. This program also targets new ways to achieve molecular
recognition, complexation and catalysis. Overall, the primary goal is
to synthesise such complex molecules in a practical manner.
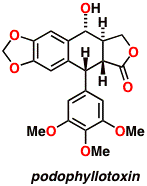
Lignans like podophyllotoxin have cancer-fighting properties and are used in chemotherapy. An efficient and highly modular approach for the synthesis of these compounds has been developed, culminating in several total syntheses including that of podophyllotoxin. This strategy has several advantages over previous syntheses, the most significant of which being that it allows a high level of convergency at the end of the synthetic route. Biological evaluations of many of our anti-cancer compounds are presently being carried out within Australia and the USA.
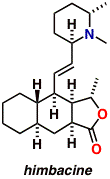 Himbacine is natural product isolated from Galbulimima baccata,
a species of tree found in Northern Australia and Papua New Guinea.
Himbacine was found to exhibit strong, selective binding to muscarinic
receptors of the M2 subtype. Speculation that selective presynaptic
muscarinic receptor antagonists might find application in the
treatment of neurodegenerative disorders such as Alzheimer’s
disease provoked our interest in Galbulimima alkaloids. A modular
total synthesis of himbacine was completed this year. The synthesis
draws upon new chemistry developed in other projects currently under
way in the group. A dozen new structural variants of himbacine are
currently undergoing biological testing with collaborators at the
University of Melbourne.
(with J. Fischer, A.J. Scott, L.A. Sharp, L.S.-M. Wong, and
F.J. Mitchelson [U. Melbourne], A.J. Reynolds [U. Sydney])
Himbacine is natural product isolated from Galbulimima baccata,
a species of tree found in Northern Australia and Papua New Guinea.
Himbacine was found to exhibit strong, selective binding to muscarinic
receptors of the M2 subtype. Speculation that selective presynaptic
muscarinic receptor antagonists might find application in the
treatment of neurodegenerative disorders such as Alzheimer’s
disease provoked our interest in Galbulimima alkaloids. A modular
total synthesis of himbacine was completed this year. The synthesis
draws upon new chemistry developed in other projects currently under
way in the group. A dozen new structural variants of himbacine are
currently undergoing biological testing with collaborators at the
University of Melbourne.
(with J. Fischer, A.J. Scott, L.A. Sharp, L.S.-M. Wong, and
F.J. Mitchelson [U. Melbourne], A.J. Reynolds [U. Sydney])
The Diels–Alder reaction is one of the most powerful and most commonly used reactions in synthetic organic chemistry. Predicting, controlling and explaining the stereochemical outcome of its intramolecular variant continues to be a major activity within the group. The location of transition structures at high levels of theory is providing stimulating new insights into the reaction. This deeper understanding is driving the development of new methodology. We have developed a novel, efficient and very general way to produce complex polycyclic molecules with useful biological properties from simple, unsaturated, acyclic precursors using sequences of Diels–Alder reactions. (with T.N. Cayzer, L. Kwan, N. Miller, A. Payne, E. Pearson, R. Tripoli, C.I. Turner, and M.N. Paddon–Row [U. NSW])

A hydrogen bond (bronze) improves the stereoselectivity of a Diels–Alder reaction.
Research in this area is concerned with the design and synthesis of host molecules based upon cavitands (rigid, bowl-shaped molecules) for molecular recognition, complexation and catalysis. Investigations into potential uses of these intriguing hosts as molecule-sized devices is under way. (with E.S. Barrett, N. Kanizaj, D.J. Sinclair, and J.R. Hansen [U. Sydney])
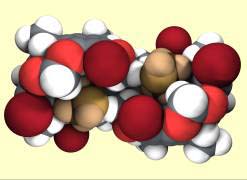
A crystal structure of one of our double-cavity hosts binding two ethanol molecules (bronzed)