Inorganic Chemistry
 Our work covers a wide range of topics in coordination and
organometallic chemistry. Particular foci include unsaturated ligands
involving metal-carbon multiple bonding and the interface of
transition and main group chemistries. In attempting to understand and
ideally control the reactivity of such systems, the nature of the
metal centre is of paramount importance and this may be tuned through
variations in oxidation state, d-configuration and most importantly
the nature of the co-ligands. Accordingly considerable effort is
directed towards the synthesis of new co-ligands which themselves do
not directly take part in ligand transformations but may moderate
these indirectly. This work is currently centred on two classes of
ligands; polythiamacrocycles and poly(methimazolyl)chelates.
Our work covers a wide range of topics in coordination and
organometallic chemistry. Particular foci include unsaturated ligands
involving metal-carbon multiple bonding and the interface of
transition and main group chemistries. In attempting to understand and
ideally control the reactivity of such systems, the nature of the
metal centre is of paramount importance and this may be tuned through
variations in oxidation state, d-configuration and most importantly
the nature of the co-ligands. Accordingly considerable effort is
directed towards the synthesis of new co-ligands which themselves do
not directly take part in ligand transformations but may moderate
these indirectly. This work is currently centred on two classes of
ligands; polythiamacrocycles and poly(methimazolyl)chelates.

There has been enormous growth in recent years in the synthesis of
compounds with two metal centres linked solely by a chain of carbon
atoms. However, this work has focused almost exclusively on even
numbered carbon chains, i.e., LnM(C≡C)MLn.
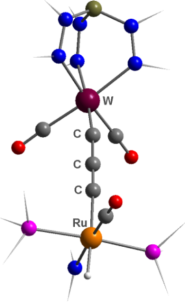
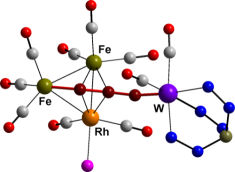 We have now developed
a route to bimetallics spanned by a three-carbon (tricarbido) chain,
LnM≡C–C≡C–MLn (see right), that
should have wide applicability. This is based on the
fluorodesilylation of silypropargylidyne complexes of the group 6
metals, and trapping of the desilylated species with a range of
transition metal complexes. The resulting bimetallic complexes serve
as precursors to polymetallic species of higher nuclearity through
coordination of the localised –C≡C- triple bond to further metal centres
(see left).
(with R. Dewhurst, M.K. Smith, A.C. Willis)
We have now developed
a route to bimetallics spanned by a three-carbon (tricarbido) chain,
LnM≡C–C≡C–MLn (see right), that
should have wide applicability. This is based on the
fluorodesilylation of silypropargylidyne complexes of the group 6
metals, and trapping of the desilylated species with a range of
transition metal complexes. The resulting bimetallic complexes serve
as precursors to polymetallic species of higher nuclearity through
coordination of the localised –C≡C- triple bond to further metal centres
(see left).
(with R. Dewhurst, M.K. Smith, A.C. Willis)
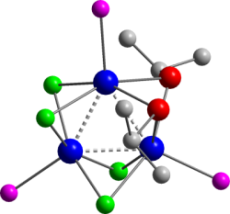
Selenoalkynes, R-C≡C-SeR’ and
alkynylselenolates R-C≡C-Se–
offer a number of potential points of reactivity in combination with
transition metals. We have been investigating the organometallic
chemistry of such species, in parallel with that of isoelectronic
isoselenacyanates, R-N=C=Se. In both cases the C-Se bonds have been
found to be particularly fragile, facilitating either intramolecular
rearrangments, or complete fragmentation. Thus, e.g., the
reaction of [Ru2Cl4(η6-cymene)2]
with Ph-C≡CSeiPr and
PCy3 was found to provide both the vinylidene complex
[RuCl2(=C=CHPh)(PCy3)2] and the
unusual cluster [Ru3(µ-SeiPr)2(µ-Cl)4(PCy3)3]
(shown) by cleavage of the C–Se bond.
(with L.M. Caldwell, M.K. Smith, A.C. Willis)
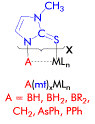 We have been investigating a range of ‘scorpionate’ ligands
in which a bridgehead group
(‘A’)
bound to two or three (‘x‘) methimazolyl
heterocycles (‘mt’)
acts as a chelate to a metal centre (‘MLn’)
[ARC Grant DP034270]. Of particular interest is the possibility of
transannular interactions between the bridgehead group, A, and the
metal. This may be simple dative coordination in the case of A = PPh,
however for A = CH2, BH2, the question of
agostic bonding arises. This is of particular relevance to the
formation of metallaboratranes via B–H activation.
We have been investigating a range of ‘scorpionate’ ligands
in which a bridgehead group
(‘A’)
bound to two or three (‘x‘) methimazolyl
heterocycles (‘mt’)
acts as a chelate to a metal centre (‘MLn’)
[ARC Grant DP034270]. Of particular interest is the possibility of
transannular interactions between the bridgehead group, A, and the
metal. This may be simple dative coordination in the case of A = PPh,
however for A = CH2, BH2, the question of
agostic bonding arises. This is of particular relevance to the
formation of metallaboratranes via B–H activation.
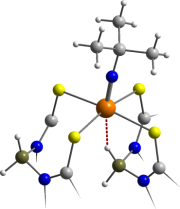
 The
synthesis of a wide range of complexes featuring such interactions
has been achieved for the metals ruthenium, tungsten, rhodium,
titanium and molybdenum. The potential hemilability of these
B–H–M
interactions is currently under investigation. The allyl complex [W
(η-C3H5)(CO)2{H2B(mt)2}]
is depicted (left) with the agostic B–H–W
interaction shown in red.
The
synthesis of a wide range of complexes featuring such interactions
has been achieved for the metals ruthenium, tungsten, rhodium,
titanium and molybdenum. The potential hemilability of these
B–H–M
interactions is currently under investigation. The allyl complex [W
(η-C3H5)(CO)2{H2B(mt)2}]
is depicted (left) with the agostic B–H–W
interaction shown in red.
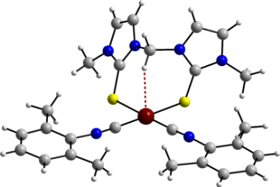
The imido complex [Ti(=NCMe3){H2B(mt)2}2] is unusual in that it displays two distinct coordination modes for the H2B(mt)2 ligands; one simply acts as a bidentate chelate whilst the second also involves the formation of an agostic B–H–Ti interaction (shown in red, right).
The facile formation of agostic B–H–metal interactions
for H2B(mt)2 ligands is in part due to the
favourable geometry. We therefore investigated whether such a geometry
might induce agostic interactions based on elements other than
boron.
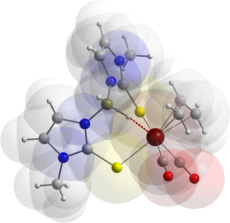
 This is indeed the case as illustrated by the salt
[Rh(CNC6H3Me2)2{H2C(mt)2}]BF4
in which the bridgehead CH2 group is weakly coordinated to
both the rhodium centre (shown in red) and the counteranion.
(with R.J. Abernethy, E.R. Humphrey, H. Neumann, M.K. Smith, N.
Tshabang, A.C. Willis)
This is indeed the case as illustrated by the salt
[Rh(CNC6H3Me2)2{H2C(mt)2}]BF4
in which the bridgehead CH2 group is weakly coordinated to
both the rhodium centre (shown in red) and the counteranion.
(with R.J. Abernethy, E.R. Humphrey, H. Neumann, M.K. Smith, N.
Tshabang, A.C. Willis)