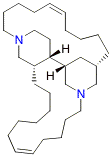
 Haliclonacyclamine A, a marine alkaloid derived from sponge found
on the Great Barrier Reef
Haliclonacyclamine A, a marine alkaloid derived from sponge found
on the Great Barrier Reef
Organic Chemistry
 The
group’s activities continue to be focused on the development of
new synthetic strategies and methodologies. The application of these
in the total synthesis of biologically active natural products and
various analogues represents another ongoing theme as does the
exploitation of novel starting materials for the same purposes. Of
particular note is the preparation, in collaboration with Dr Gregg
Whited of Genencor International Inc (Palo Alto, CA), of new,
genetically engineered forms of the bacteria E. coli that are
capable of effecting novel whole-cell biotransformations of various
poly-substituted aromatics. The metabolites resulting from such
processes are not only enantiomerically pure but also sufficiently
rich in functionality that they can serve as important new starting
materials in our synthesis program. Aspects of our work are funded by
Australian companies. For example, an APA(I)-funded PhD scholar has
recently completed his collaborative studies with Biota Holdings and
another has just started and will continue work in the same area.
Progen Industries, a Brisbane-based biotech company, is continuing to
fund two post-Doctorals who have been working on a very enjoyable
collaborative project focused on novel carbohydrate chemistries.
Collaborations with the Melbourne-based company Cytopia have also
commenced. The Alexander von Humboldt Foundation is providing Feodor
Lynen Fellowships to two German postdoctorals working in the group.
Ecological and other evaluations of analogues of the marine natural
product haliclonacyclamine A
The
group’s activities continue to be focused on the development of
new synthetic strategies and methodologies. The application of these
in the total synthesis of biologically active natural products and
various analogues represents another ongoing theme as does the
exploitation of novel starting materials for the same purposes. Of
particular note is the preparation, in collaboration with Dr Gregg
Whited of Genencor International Inc (Palo Alto, CA), of new,
genetically engineered forms of the bacteria E. coli that are
capable of effecting novel whole-cell biotransformations of various
poly-substituted aromatics. The metabolites resulting from such
processes are not only enantiomerically pure but also sufficiently
rich in functionality that they can serve as important new starting
materials in our synthesis program. Aspects of our work are funded by
Australian companies. For example, an APA(I)-funded PhD scholar has
recently completed his collaborative studies with Biota Holdings and
another has just started and will continue work in the same area.
Progen Industries, a Brisbane-based biotech company, is continuing to
fund two post-Doctorals who have been working on a very enjoyable
collaborative project focused on novel carbohydrate chemistries.
Collaborations with the Melbourne-based company Cytopia have also
commenced. The Alexander von Humboldt Foundation is providing Feodor
Lynen Fellowships to two German postdoctorals working in the group.
Ecological and other evaluations of analogues of the marine natural
product haliclonacyclamine A
 Haliclonacyclamine A, a marine alkaloid derived from sponge found
on the Great Barrier Reef
Haliclonacyclamine A, a marine alkaloid derived from sponge found
on the Great Barrier Reef
|
have been undertaken in collaboration with A/Professor Mary Garson of the University of Queensland while related assessments of the two enantiomeric forms of natural product aiphanol are being undertaken in a joint venture involving Professor Chris Parish of the JCSMR (ANU), Dr Paul Savage of CSIRO Molecular Science and Professor Gerd Dannhardt of the University of Mainz in Germany. A PhD student from Professor Armin de Meijere’s group at the University of Göttingen has spent six months in our labs working on a collaborative project directed towards the synthesis of steroids, whilst two students from Professor Hans Reissig’s group at the Free University in Berlin each stayed in our labs for one month working on the exploitation of pyrroles as scaffolds in natural products synthesis. |
Research highlights in 2003 include:
|
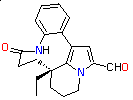 Rhazinal, a terrestrially-derived alkaloid exhibiting potent anti-mitotic
properties
Rhazinal, a terrestrially-derived alkaloid exhibiting potent anti-mitotic
properties
|
In the methodological area, development of palladium[0]-catalysed Ullmann cross-coupling methods for the regiocontrolled synthesis of indoles and other heterocycles has been another important activity. Patents relating to this work have or are being filed.
The title compounds, which can be obtained by enantioselective microbial oxidation of the corresponding arene or through manipulation of the shikimic acid biosynthetic pathway, continue to serve as important starting materials for the preparation of a structurally diverse array of poly-oxygenated natural products and their analogues. Methods for the enantiodivergent elaboration of cis-1,2-dihydrocatechols through Diels–Alder chemistry has been a major area of activity and the adducts derived from such processes have been converted, using photochemical processes, into the polycyclic skeleta associated with a diverse range of terpenoid natural products. Other natural products being targeted include the alkaloids galanthamine (a useful agent for the treatment of Alzhiemer’s disease), brunsvigine and vindoline (a clinically important anti-cancer agent) as well as the macrolide tricholomenyn B (and potent anti-mitotic agent), the fungal metabolite diversonol and the plant-growth regulating substance cladospolide B. The preparation of various rare sugars together with certain sugar mimetics has been another activity in this area and one that has been carried out with commercial partners. (with K. Austin, M. Bonnet, C. Chun, M. Essers, M. Friend, P. Guan, G.J. Harfoot, N.L. Hungerford, J. Jury, O.P. Karunaratne, D.T.J. Loong, D.W. Lupton, X.H. Ma, J. Renner, and R.H. Don. V. Ferro [Progen Industries Ltd, Brisbane], J.N. Lambert [Biota Pty Ltd, Melbourne], G.M. Whited [Genencor International Inc, Palo Alto])