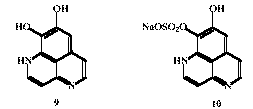Go to 2002 RSC Annual Report Index
Go to 2002 RSC Annual Report Index
Organic Chemistry
Organic Synthesis
Professor Lew Mander
http://rsc.anu.edu.au/research/mander.php
Our research interests encompass two major overlapping themes. The first of these is focused on methods and strategies for the synthesis of complex natural products that have interesting biological properties, while the second is concerned with the molecular basis of plant growth regulation, using organic synthesis as an enabling technology. Within this context, members of the group have successfully completed syntheses of numerous complex natural products and developed a number of useful synthetic procedures. The second major activity in the group is concerned with natural plant bioregulators with special reference to the gibberellins (GAs). GAs effect numerous aspects of plant growth and development, for example, germination, induction of stem growth and flowering, and there are several commercially valuable applications. Studies pursued in collaboration with groups in the CSIRO and the University of Calgary have led to the discovery of semi-synthetic derivatives that interfere with the plant's natural production of phytohormones, thereby inhibiting growth.
Structural and Synthetic Studies on New Gibberellins
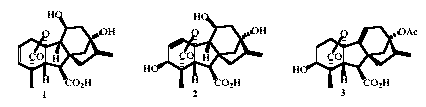
There are now approximately one hundred and thirty confirmed naturally occurring gibberellins, with tentative assignments of structure for a further ten or so. Most of the recently isolated compounds have been obtained in trace amounts and so the usual tools for structure elucidation cannot be deployed. Fortunately, it is possible to make educated guesses regarding the new structures from mass spectra and then to confirm the assignments by synthesis from one of the more readily available fungal GAs. Over the past year, methodology has been developed for the preparation of several 11,13-dihydroxy-GAs. These GAs display a characteristic signature in mass spectra of a base peak at m/z 296 plus a strong peak at m/z 239 and has allowed the identification of two new GAs, one from loquat fruit (11b-hydroxy-GA20 1) and the other from strawberries (11ß-hydroxy-GA3 2).A pivotal step in these syntheses is the hydroboration of the 9(11),16-diene function in 13-acetoxy-GAs, e.g. 3. (with E.J. Beck, J.R. Crow, O.E. Hutt, T.P. Le, and B. Twitchin)
See also : http://www.plant-hormones.info/gibberellins.htm
Total Synthesis of Natural Products
Synthetic studies are being directed towards the assembly of several highly caged natural products. They include members of an unusual group of alkaloids isolated from the Northern Australian rain forest species, Galbulimima belgraveana. One compound, himbacine, is a potent muscarinic antagonist and is a lead compound in the search for drugs to treat Alzheimer's disease. Our own efforts have been focused on the more complex members of the family, for example, himandridine 4 and himaline (GB 13) 5. The synthesis of 5 has been achieved and the full hexacyclic skeleton of 4 assembled.
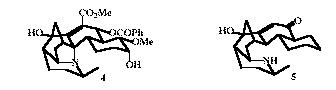
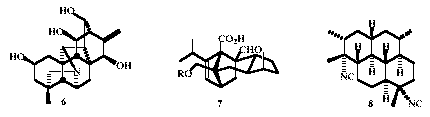
Preliminary
studies on the construction of the heptacyclic family of diterpenoid
alkaloids typified by hetisine 6 have been undertaken. A
number of promising leads have been developed, but have failed
because of the intervention of unusual and unprecedented
rearrangements. An enantio-convergent synthesis of the anti-fungal
agent sordaricin 7 has been completed, while a promising
beginning has been towards the total synthesis of the anti-malarial
diterpenoid, diisocyanoadociane 8. (with K.A. Fairweather,
M.M.W. McLachlan, P.D. O'Connor, R.J. Thomson)
Isolation of New Natural Products from the Marine Sponge Aaptos aaptos
Two new marine alkaloids have been isolated by reverse phase HPLC from methanolic extracts of the marine sponge Aaptos aaptos collected from the Bunaken Sea Garden, North Sulawesi, Indonesia. 1H and 13C NMR together with mass spectroscopic studies established the major compound as 8,9-dihydroxy-1H-benzo[de][1,6]naphthyridine 9 (isolated as the trifluoroacetate salt) and the derived sulfonate 10. The parent compound was synthesised earlier by Steglich et al. and has been proposed as a biosynthetic precursor of three marine alkaloids previously isolated from Aaptos. These compounds, which were also isolated from our Aaptos extract, show antagonistic effects on a-adrenergic receptors as well as anti-tumour activity. (with A.J. Herlt, and W.A.R. Romberg, R.J. Rumampuk [State U. Manado, Indonesia], W. Steglich, Ludwig-Maximilians-U., Munich, Germany])