
 Go to 2002 RSC Annual Report Index
Go to 2002 RSC Annual Report Index
Inorganic Chemistry
Inorganic Stereochemistry and Asymmetric Synthesis
Professor Bruce Wild
http://rsc.anu.edu.au/research/wild.php
Coordination chemistry has merged with organic and organometallic chemistry and with catalysis with the result that there are now modifications available for nearly every standard reaction for converting achiral organic precursors into chiral products. Together with modern purification techniques, this has allowed the preparation - in a single step - of compounds in >98% enantiomeric purity for many reaction types. Central to the design of the catalysts for homogeneous asymmetric syntheses has been the synthesis of enantiomerically pure phosphines, which act as the auxiliaries, in conjunction with appropriate metals, for transmitting the chiral information to the products. There are now industrial processes employing chiral phosphine - transition metal catalysts. Because the biological activity of one enantiomer of a substance can differ completely from that of the mirror-image substance, the pharmaceutical industry pays careful attention to the separation and purity of enantiomers of chiral drugs. Work in this group is concerned with the synthesis of new types of chiral ligands, especially enantiomerically pure phosphines and arsines, for use as probes of inorganic stereochemistry, rearrangements in metal complexes, and as auxiliaries for asymmetric synthesis.
Professor Wild visited Germany as a Re-invited Alexander von Humboldt Fellow during September-November where he was based at the Technical University Munich. During this period, he presented an invited lecture at the retirement symposium for Professor Ekkehard Lindner at the University of Tübingen and gave research lectures at the Technical University Munich, the Ludwig-Maximilians-University Munich, the University of Leipzig, and the École Polytechnique, Paris. A postgraduate student from the University of Leipzig, Robert Wolf, worked in the group during July-August on a grant funded by the Deutscher Akademischer Austausch Dienst e.V. (DAAD).
Alkoxyphosphonium Salts
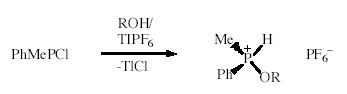
Two-coordinate phosphenium ions are stabilised by phosphines and pyridines. We have found that alcohols also react with phosphenium salts to give alkoxyhydrophosphonium salts that can be isolated as colourless, crystalline solids; two of the salts have been characterised by X ray crystallography. Interestingly, with t-butanol the derivative rapidly undergoes ß-hydrogen elimination to give isobutylene and a dihydrophosphonium salt. (with J.W. Wielandt, A.C. Willis)
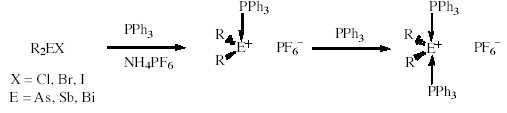
Group 15 Element Coordination Complexes
Recently, a series of air- and water-stable tertiary phosphine-stabilised arsenium salts of the general type [R3P -> AsR2]PF6 has been isolated. In the solid state, the stereochemistry around the arsenic in these complexes is a trigonal pyramid in which the phosphorus atom occupies the apical position, orthogonal to the plane of the trigonal sp2 arsenium ion. By transferring the concept of preparation to the higher homologues of arsenic, the respective stibenium and bismuthenium cations stabilised by triphenylphosphine have been established. These novel group 15 element coordination complexes are colourless, crystalline compounds showing a higher sensitivity towards oxygen and moisture than the respective arsenium salts. Moreover, in contrast to arsenic, an excess of phosphine ligand affords stibenium and bismuthenium salts in which two ligands coordinate to the cationic centre. This behaviour seems to be in line with the increase of the ionic radii when going from arsenic via antimony to bismuth. We are currently investigating the electronic effects around the cationic centres of these compounds by means of theoretical calculations. (with A.J. Edwards, K.A. Porter, J.W. Wielandt, A.C. Willis, J. Zank, and R.A. Stranger [Dept. Chemistry, ANU])
Alkyne Exchange in Phosphirenium Ions
Phosphirenium
salts undergo alkyne-exchange reactions, reminiscent of the
alkyne-exchange behaviour of transition metal ions. A kinetic study
of the exchange reaction has shown the rate to be independent of the
alkyne concentration, suggesting that the key step in the exchange is
the dissociation of the alkyne unit from the phosphorus to form a
transient phosphenium ion. Such a finding supports the formulation
of phosphirenium salts as alkyne -> phosphenium
donor-acceptor complexes-a notion whose generality and synthetic
utility we are now applying to a range of p-block elements.
(with N.E. Brasch, I.G. Hamilton,
E.H. Krenske, A.C. Willis)
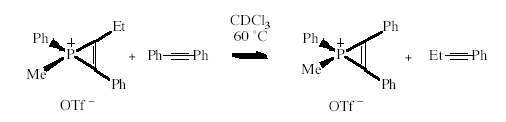
Halide Complexes of Stibenium Ions
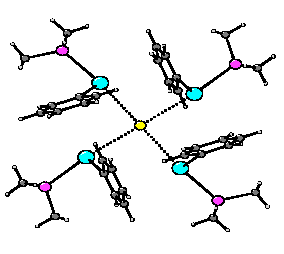 The
coordination behaviour of the heavier Group 15 elements has recently
been developed through the synthesis of many adducts of the type
The
coordination behaviour of the heavier Group 15 elements has recently
been developed through the synthesis of many adducts of the type[R3P -> ER1R2]PF6 (R3P = Me3P, Me2PhP, MePh2P, Ph3P; E = P, As, Sb, Bi; R1, R2 = Me, Ph). All crystallographically-characterised examples are monomeric in the solid state. The geometry of the E atom is that of a trigonal pyramid: the substituents R1 and R2 and the lone pair surround the E atom in a trigonal plane, while the phosphine coordinates in an apical position. During the course of this work we have encountered, for E = Sb, a second class of compound-halide complexes with the formula
{[Me3P -> SbPh2]4X}(PF6)3 (X = Cl, Br). The molecular structures of these complexes (shown, for X = Cl) reveal an unusual square-planar arrangement of Sb atoms around the halide. We are currently investigating the factors dictating the stability and stereochemistry of these aggregates, including a study of halide selectivity.
(with R.D. Dewhurst, A.J. Edwards, E.H. Krenske, K.A. Porter, J.W. Wielandt, A.C. Willis)
Stereoselective Synthesis of Two-bladed Propeller Octahedral Metal Complexes
Modern organic synthesis is at the level of an art form, with most types of organic compounds now being available as single diastereomers or enantiomers following highly stereoselective and frequently catalytic reactions; inorganic synthesis by comparison has changed little in one hundred years - most metal complexes are still prepared as mixtures of stereoisomers that require tedious separations and resolutions by traditional methods. We have embarked on a program aimed at demonstrating that chiral metal complexes can be prepared by asymmetric synthesis - inorganic asymmetric synthesis. The approach being adopted is to transfer chiral information to a metal centre by means of an enantiomerically pure chiral auxiliary attached to appropriate chelating agents, as in 1, with the auxiliary group subsequently being removed to leave the configurationally pure metal complex, chiral at the metal alone. For this method to be successful, the product must have sufficient chemical stability to withstand the conditions of the synthesis and configurational stability to observe the single enantiomer produced. For these reasons, we have chosen two-bladed propeller complexes of the types (±)-[M(PAPHY)2]X2 and (±)-[M(PAPY)2], which are available for a wide range of metals and for which the nickel(II) and zinc(II) complexes have been resolved. (with R.J. Warr, A.C. Willis)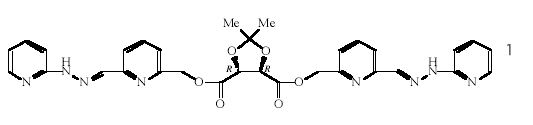
Stabilisation of Double alpha-Helix Conformers of Dinuclear Metal Helicates Containing Tetra(tertiary phosphines)
The self-assembly of molecules into large supramolecular structures is an important feature in biology and is now readily achieved in inorganic coordination chemistry with appropriate helicating ligands and metal ions. Previous work in our laboratory has shown that (S,S)-tetraphos spontaneously self-assembles dinuclear metal helicates of the type (M)-[M2{(R,R)-tetraphos}2](PF6)2 upon reaction with univalent silver and gold salts. The d or l twist of the central 10-membered ring containing the two metal ions, which has the chiral twist boat-chair-boat conformation, generates the double a-helix or side-by-side parallel helix conformer of the helicate. 1H NMR spectroscopic investigations indicated rapid interconversion between the two conformers in solution, although in the solid state the silver helicate crystallises with both conformers in the unit cell and the gold helicate with the more compact side-by-side conformer alone. A related double-stranded tricopper(I) helicate of a configurationally pure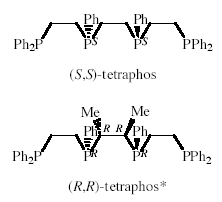
hexa(tertiary
phosphine) has recently been synthesised and characterised by X-ray
crystallography. Current work is focused on the synthesis of
(R,R)-tetraphos*, which molecular modelling
suggests will react with univalent Group 11 salts to produce
stereoselectively the double a-helix
conformers of the dinuclear metal helicates. (with P.A. Gugger,
H.J. Kitto)